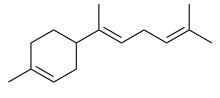
 α-Bisabolene | |
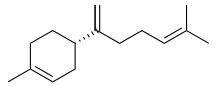 β-Bisabolene | |
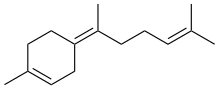 γ-Bisabolene | |
| Names | |
|---|---|
| IUPAC names
(α): (E)-1-Methyl-4-(6-methylhepta-2,5-dien-2-yl)cyclohex-1-ene (β): (S)-1-Methyl-4-(6-methylhepta-1,5-dien-2-yl)cyclohex-1-ene (γ): (Z)-1-Methyl-4-(6-methylhept-5-en-2-ylidene)cyclohex-1-ene | |
| Identifiers | |
| |
3D model (JSmol) |
|
| α: 2414203 β: 2044625 γ: 2501191 | |
| ChEBI |
|
| ChemSpider | |
| KEGG |
|
PubChem CID |
|
| UNII |
|
| |
| |
| Properties | |
| C15H24 | |
| Molar mass | 204.357 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Bisabolenes are a group of closely related natural chemical compounds which are classified as sesquiterpenes. Bisabolenes are produced from farnesyl pyrophosphate (FPP)[1] and are present in the essential oils of bisabol, and of a wide variety of other plants including cubeb, lemon, and oregano. Various derivates also function as pheromones in different insects, such as stink bugs[2] and fruit flies.[3] Bisabolenes are produced by several fungi, though their biological role in that group of organisms remains unclear.[4]
Three isomers are known, α-, β-, and γ-bisabolene,[5][6] which differ by the positions of the double bonds.
Uses
Bisabolenes are intermediates in the biosynthesis of many other natural chemical compounds,[7] including hernandulcin, a natural sweetener. β-Bisabolene has a balsamic odor[8] and is approved in Europe as a food additive.
Bisabolene has been identified as a biologically producible precursor to a diesel fuel alternative and/or cold weather additive bisabolane. [9]
See also
References
- ↑ "MetaCyc bisabolene biosynthesis (engineered)". biocyc.org. Retrieved 2018-05-28.
- ↑ Aldrich, J.R.; Numata, H.; Borges, M.; Bin, F.; Waite, G.K.; Lusby, W.R. (1993). "Artifacts and pheromone blends from Nezara spp. and other stink bug (Heteroptera: Pentatomidae)". Zeitschrift für Naturforschung. 48C (1–2): 73–79. doi:10.1515/znc-1993-1-214. S2CID 40523228. Archived from the original on 2013-04-08. Retrieved 2013-10-22.
- ↑ Lu, F.; Teal, P.E. (2001). "Sex pheromone components in oral secretions and crop of male Caribbean fruit flies, Anastrepha suspensa (Loew)". Archives of Insect Biochemistry and Physiology. 48 (3): 144–154. doi:10.1002/arch.1067. PMID 11673844.
- ↑ Spakowicz, Daniel J.; Strobel, Scott A. (2015). "Biosynthesis of hydrocarbons and volatile organic compounds by fungi: bioengineering potential". Applied Microbiology and Biotechnology. 99 (12): 4943–4951. doi:10.1007/s00253-015-6641-y. PMC 4677055. PMID 25957494.
- ↑ "pubchem/alpha-Bisabolene".
- ↑ "pubchem/beta-Bisabolene".
- ↑ Bisabolene derived sesquiterpenoid biosynthesis Archived November 2, 2010, at the Wayback Machine
- ↑ (−)-β-bisabolene, flavornet.org
- ↑ "Alternative Diesel Fuel from Biosynthetic Bisabolene". 13 August 2014.
External links
- Beta-bisabolene, NIST Chemistry WebBook listing