 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,2-Dimethylhydrazine[1] | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | SDMH[2] |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.149.162 |
| KEGG | |
| MeSH | 1,2-Dimethylhydrazine |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2382 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties[3] | |
| C2H8N2 | |
| Molar mass | 60.100 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | Ichtyal, ammoniacal |
| Density | 827.4 kg m−3 (at 20 °C) |
| Melting point | −9 °C (16 °F; 264 K) |
| Boiling point | 87 °C; 188 °F; 360 K |
| Miscible | |
| Thermochemistry | |
Heat capacity (C) |
171.04 J K−1 mol−1 |
Std molar entropy (S⦵298) |
199.15 J K−1 mol−1 |
Std enthalpy of combustion (ΔcH⦵298) |
−1987–−1978 kJ mol−1 |
| Hazards | |
| GHS labelling:[4] | |
 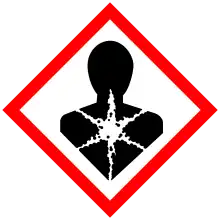 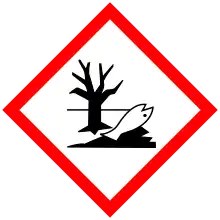 | |
| Danger | |
| H301, H311, H331, H350, H411 | |
| P201, P202, P261, P264, P270, P271, P273, P280, P281, P301+P310, P302+P352, P304+P340, P308+P313, P311, P312, P322, P330, P361, P363, P391, P403+P233, P405 | |
| Related compounds | |
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
symmetrical dimethylhydrazine, or 1,2-dimethylhydrazine, is the organic compound with the formula (CH3NH)2. It is one of the two isomers of dimethylhydrazine. Both isomers are colorless liquids at room temperature, with properties similar to those of methylamines. Symmetrical dimethylhydrazine is a potent carcinogen that acts as a DNA methylating agent.[5][6] The compound has no commercial value, in contrast to its isomer unsymmetrical dimethylhydrazine (1,1-dimethylhydrazine), which is used as a rocket fuel.[7]
It is used to induce colon tumors in experimental animals - particularly mice and feline cell samples.[1][8][9]
References
- 1 2 1,2-Dimethylhydrazine from PubChem
- 1 2 3 4 74-79-3 1,2-Dimethylhydrazine
- ↑ Record of 1,2-Dimethylhydrazin in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 21 March 2008.
- ↑ "1,2-Dimethylhydrazine". pubchem.ncbi.nlm.nih.gov. Retrieved 22 December 2021.
- ↑ Salehi, Alireza; Hosseini, Seyed Mohammad; Kazemi, Sohrab (2022-06-23). "Antioxidant and Anticarcinogenic Potentials of Propolis for Dimethylhydrazine-Induced Colorectal Cancer in Wistar Rats". BioMed Research International. 2022: e8497562. doi:10.1155/2022/8497562. ISSN 2314-6133. PMC 9246617. PMID 35782078.
- ↑ Valaei, Amirhasan; Azadeh, Fatemeh; Mostafavi Niaki, Seyedeh Talayeh; Salehi, Alireza; Shakib Khoob, Maede; Mirebrahimi, Seyed Hesam odin; Kazemi, Sohrab; Hosseini, Seyed Mohammad (2022-10-10). "Antioxidant and Anticancer Potentials of the Olive and Sesame Mixture against Dimethylhydrazine-Induced Colorectal Cancer in Wistar Rats". BioMed Research International. 2022: e5440773. doi:10.1155/2022/5440773. ISSN 2314-6133. PMC 9576397. PMID 36262974.
- ↑ Gangadhar Choudhary, Hugh Hansen (1998). "Human health perspective of environmental exposure to hydrazines: A review". Chemosphere. 37 (5): 801–843. Bibcode:1998Chmsp..37..801C. doi:10.1016/S0045-6535(98)00088-5. PMID 9717244.
- ↑ Cruse, J. P.; Lewin, M. R.; Ferulano, G. P.; Clark, C. G. (1978). "Co-carcinogenic effects of dietary cholesterol in experimental colon cancer". Nature. 276 (5690): 822–5. Bibcode:1978Natur.276..822C. doi:10.1038/276822a0. PMID 723955. S2CID 4303843.
- ↑ Wijnands, M.V.W. (1999). "A comparison of the effects of dietary cellulose and fermentable galacto-oligosaccharide, in a rat model of colorectal carcinogenesis: fermentable fibre confers greater protection than non-fermentable fibre in both high and low fat backgrounds". Carcinogenesis. 20 (4): 651–6. doi:10.1093/carcin/20.4.651. PMID 10223195.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.