 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,2-Dioxane | |
| Other names
o-Dioxane | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C4H8O2 | |
| Molar mass | 88.106 g·mol−1 |
| Appearance | colorless liquid |
| Boiling point | 116–117 °C (241–243 °F; 389–390 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
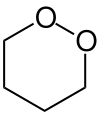
1,2-Dioxane or o-dioxane is an organic compound with the molecular formula (CH2)4O2, classified as a cyclic peroxide. Its synthesis was reported in 1956 by Criegee and Müller, who prepared it by reacting butane-1,4-diol bis(methanesulfonate) with hydrogen peroxide and distilled it as a colorless liquid. Acids and bases decompose it to gamma-hydroxybutyraldehyde.[1]
Substituted 1,2-dioxanes have also been prepared, and some have been isolated from natural sources.[2]
See also
References
- ↑ Criegee, Rudolf; Müller, Gerhard (1956). "1,2-Dioxan". Chem. Ber. 89 (2): 238–240. doi:10.1002/cber.19560890209.
- ↑ Parrish, Jonathan D.; Ischay, Michael A.; Lu, Zhan; Guo, Song; Peters, Noel R.; Yoon, Tehshik P. (2012). "Endoperoxide Synthesis by Photocatalytic Aerobic [2 + 2 + 2] Cycloadditions". Organic Letters. 14 (6): 1640–1643. doi:10.1021/ol300428q. PMC 3306464. PMID 22372647.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.