 | |
 | |
| Names | |
|---|---|
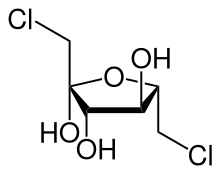
| IUPAC name
(3S,4S,5S)-1,6-dichloro-3,4,5-trihydroxyhexan-2-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H10Cl2O4 | |
| Molar mass | 217.04 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
1,6-Dichloro-1,6-dideoxyfructose (dichlorodideoxyfructose)[1][2][3] is chlorinated derivative of the sugar fructose. It is one of the two components believed to comprise the disaccharide sucralose,[4] a commercial sugar substitute.
Metabolism
In mammals, 1,6-dichloro-1,6-dideoxyfructose is metabolized in the liver and erythrocytes by a reaction with glutathione that replaces one of the chlorine atoms, forming 6-chlorofructos-1-yl glutathione (or chlorofructosyl glutathione).[5]
References
- ↑ Hough, L. (1993), Khan, Riaz (ed.), "High-intensity, low-calorie sweeteners", Low-Calorie Foods and Food Ingredients, Boston, MA: Springer US, pp. 138–164, doi:10.1007/978-1-4615-3114-2_7, ISBN 978-1-4615-3114-2, retrieved 2022-11-24
- ↑ PubChem. "1,6-Dichloro-1,6-dideoxyfructose". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-11-24.
- ↑ "1,6-dichloro-1,6-dideoxyfructose | C6H10Cl2O4 | ChemSpider". www.chemspider.com. Retrieved 2022-11-24.
- ↑ Labare, M. P.; Alexander, M. (1994-10-01). "Microbial cometabolism of sucralose, a chlorinated disaccharide, in environmental samples". Applied Microbiology and Biotechnology. 42 (1): 173–178. doi:10.1007/BF00170242. ISSN 1432-0614. PMID 7765816. S2CID 20916443.
- ↑ Hughes, H M; Powell, G M; Snodin, D J; Daniel, J W; Crawford, A; Sanders, J K; Curtis, C G (15 April 1989). "Glutathione-dependent dechlorination of 1,6-dichloro-1,6-dideoxyfructose". Biochemical Journal. 259 (2): 537–543. doi:10.1042/bj2590537. PMC 1138541. PMID 2719664.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.