 | |
-biphenyl-3D-balls.png.webp) | |
| Names | |
|---|---|
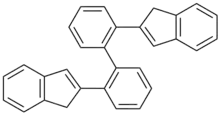
| Preferred IUPAC name
2,2′-Di(1H-inden-2-yl)-1,1′-biphenyl | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C30H22 | |
| Molar mass | 382.506 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
2,2′-Bis(2-indenyl) biphenyl is an organic compound with the formula [C6H4C9H7]2. The compound is the precursor, upon deprotonation, to ansa-metallocene complexes within the area of transition metal indenyl complexes.
Metals studied with 2,2′-bis(2-indenyl) biphenyl include titanium, zirconium, and hafnium. The ligand and its complexes have been prepared by the research group of the late Brice Bosnich at The University of Chicago.[1] Zirconium and hafnium complexes made from this ligand were found to be active catalysts for the polymerization of the smallest alkenes – compounds with carbon-carbon double bonds—namely, ethylene and propylene. The use of such complexes in the polymerization of alkenes has since been reported, and patented by DSM Research.[2][3]
References
- ↑ Ellis, W.W.; Hollis, T.K.; Odenkirk, W.; Whelan, J.; Ostrander, R.; Rheingold, A.L.; Bosnich, B. (1993). "Synthesis, Structure, and Properties of Chiral Titanium and Zirconium Complexes Bearing Biaryl Strapped Substituted Cyclopentadienyl Ligands". Organometallics. 12 (11): 4391–4401. doi:10.1021/om00035a026.
- ↑ H. J. Arts, M. Kranenburg, R. H. A. M. Meijers, E. G. Ijpeij, G. J. M. Gruter and F. H. Beijer. Indenyl Compounds for the Polymerization of Olefins US Patent 6,342,622; Jan. 29, 2002.
- ↑ E. G. Ijpeij; F. H. Beijer; H. J. Arts; C. Newton; J. G. de Vries; G. J. M. Gruter (2002). "A Suzuki Coupling Based Route to 2,2'-Bis#2-indenyl#biphenyl Derivatives" (PDF). J. Org. Chem. 67 (1): 169–176. doi:10.1021/jo016040i. PMID 11777455.