 | |
| Names | |
|---|---|
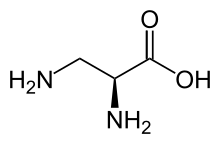
| IUPAC name
3-Amino-L-alanine | |
| Systematic IUPAC name
(2S)-2,3-Diaminopropanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H8N2O2 | |
| Molar mass | 104.109 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
2,3-Diaminopropionic acid (2,3-diaminopropionate, Dpr)[1] is a non-proteinogenic amino acid found in certain secondary metabolites, including zwittermicin A[2] and tuberactinomycin.[3]
Biosynthesis
2,3-Diaminopropionate is formed by the pyridoxal phosphate (PLP) mediated amination of serine.

Biosynthesis of l-2,3 Diaminopropionate
References
- ↑ Frenkel-Pinter M, Haynes JW, C M, Petrov AS, Burcar BT, Krishnamurthy R, et al. (August 2019). "Selective incorporation of proteinaceous over nonproteinaceous cationic amino acids in model prebiotic oligomerization reactions". Proceedings of the National Academy of Sciences of the United States of America. 116 (33): 16338–16346. doi:10.1073/pnas.1904849116. PMC 6697887. PMID 31358633.
- ↑ Rogers EW, Molinski TF (February 2007). "Asymmetric synthesis of diastereomeric diaminoheptanetetraols. A proposal for the configuration of (+)-zwittermicin a". Organic Letters. 9 (3): 437–40. doi:10.1021/ol062804a. PMC 2729442. PMID 17249781.
- ↑ Thomas MG, Chan YA, Ozanick SG (September 2003). "Deciphering tuberactinomycin biosynthesis: isolation, sequencing, and annotation of the viomycin biosynthetic gene cluster". Antimicrobial Agents and Chemotherapy. 47 (9): 2823–30. doi:10.1128/AAC.47.9.2823-2830.2003. PMC 182626. PMID 12936980.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.