 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Bromo-2-methylpropane[1] | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| 1730892 | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.333 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2342 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H9Br | |
| Molar mass | 137.020 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.22 g mL−1 (at 20 °C)[2] |
| Melting point | −16.20 °C; 2.84 °F; 256.95 K |
| Boiling point | 73.3 °C; 163.8 °F; 346.4 K |
| log P | 2.574 |
Henry's law constant (kH) |
310 nmol Pa−1 kg−1 |
Refractive index (nD) |
1.4279 |
| Thermochemistry | |
Heat capacity (C) |
165.7 J K mol−1 |
Std enthalpy of formation (ΔfH⦵298) |
−133.4 kJ mol−1 |
| Hazards | |
| GHS labelling: | |
 | |
| Danger | |
| H225 | |
| P210 | |
| Flash point | 16 °C (61 °F; 289 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
|
| Related compounds | |
Related alkanes |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
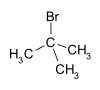
tert-Butyl bromide (also referred to as 2-bromo-2-methylpropane) is an organic compound with the formula Me3CBr (Me = methyl). The molecule features a tert-butyl group attached to a bromide substituent. This organobromine compound is used as a standard reagent in synthetic organic chemistry. It is a colorless liquid.
Reactions
It is used to introduce tert-butyl groups. Illustrative is the tert-butylation of cyclopentadiene to give di-tert-butylcyclopentadiene:[3]
- C5H6 + 2 NaOH + 2 Me3CBr → (Me3C)2C5H4 + 2 NaBr + 2 H2O
Other aspects
tert-Butyl bromide used to study the massive deadenylation of adenine based-nucleosides induced by halogenated alkanes (alkyl halides) under physiological conditions. 2-Bromo-2-methylpropane causes the massive deguanylation of guanine based-nucleosides and massive deadenylation of adenine based-nucleosides.[4]
Phase transition from orthorhombic Pmn21 phase III at low temperatures (measurements from 95 K), to a disordered rhombohedral phase II at 205-213 K. Phase II can exist from 213-223 K, partly coincident with an FCC phase I, which can be observed between 210-250 K. Phase transitions have also been studied at high pressure (up to 300MPa)[5]
References
- ↑ "2-Bromo-2-methylpropane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification. Retrieved 16 June 2012.
- ↑ CRC Handbook of Chemistry and Physics 65th Ed.
- ↑ Reiners, Matthias; Ehrlich, Nico; Walter, Marc D. (2018). "Synthesis of 1,3,5-Tri-tert-Butylcyclopenta-1,3-diene and Its Metal Complexes Na{1,2,4-(Me3C)3C5H2} and Mg{η5-1,2,4-(Me3C)3C5H2)2". Inorganic Syntheses. 37: 199. doi:10.1002/9781119477822.ch8.
- ↑ “2-Bromo-2-Methylpropane 135615.” H2NC6H4CO2C2H5, Drugs, www.sigmaaldrich.com/catalog/product/aldrich/135615?lang=en®ion=US.
- ↑ “2-Bromo-2-Methylpropane Structures.” The Cambridge Crystallographic Data Centre (CCDC), www.ccdc.cam.ac.uk/structures/search?id=doi:10.5517/ccvcqmj&sid=DataCite