 | |
| Names | |
|---|---|
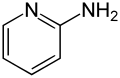
| Preferred IUPAC name
Pyridin-2-amine | |
| Other names
2-Pyridinamine; 2-Pyridylamine; α-Aminopyridine; α-Pyridylamine[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.263 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2671 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H6N2 | |
| Molar mass | 94.117 g·mol−1 |
| Appearance | colourless solid |
| Melting point | 59 to 60 °C (138 to 140 °F; 332 to 333 K) |
| Boiling point | 210 °C (410 °F; 483 K) |
| >100%[1] | |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H301, H311, H312, H315, H319, H335, H411 | |
| P261, P264, P270, P271, P273, P280, P301+P310, P302+P352, P304+P340, P305+P351+P338, P312, P321, P322, P330, P332+P313, P337+P313, P361, P362, P363, P391, P403+P233, P405, P501 | |
| Flash point | 68 °C; 154 °F; 341 K |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
200 mg/kg (rat, oral) 50 mg/kg (mouse, oral)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 0.5 ppm (2 mg/m3)[1] |
REL (Recommended) |
TWA 0.5 ppm (2 mg/m3)[1] |
IDLH (Immediate danger) |
5 ppm[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
2-Aminopyridine is an organic compound with the formula H2NC5H4N. It is one of three isomeric aminopyridines. It is a colourless solid that is used in the production of the drugs piroxicam, sulfapyridine, tenoxicam, and tripelennamine. It is produced by the reaction of sodium amide with pyridine, the Chichibabin reaction.[3]
Structure
Although 2-hydroxypyridine converts significantly to the pyridone tautomer, the related imine tautomer (HNC5H4NH) is less important for 2-aminopyridine.
Toxicity
The acute toxicity is indicated by the LD50 = 200 mg/kg (rat, oral).
References
- 1 2 3 4 5 NIOSH Pocket Guide to Chemical Hazards. "#0026". National Institute for Occupational Safety and Health (NIOSH).
- ↑ "2-Aminopyridine". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ↑ Shimizu, Shinkichi; Watanabe, Nanao; Kataoka, Toshiaki; Shoji, Takayuki; Abe, Nobuyuki; Morishita, Sinji; Ichimura, Hisao (2007). "Pyridine and Pyridine Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_399. ISBN 978-3527306732.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.