 | |||
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Bromobutanoic acid | |||
| Other names
2-Bromobutyric acid alpha-Bromobytyric acid dl-2-Bromobutyric acid α-Bromobutyricacid | |||
| Identifiers | |||
| |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.001.177 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C4H7BrO2 | |||
| Molar mass | 167.002 g·mol−1 | ||
| Appearance | colorless liquid | ||
| Density | 1.567 g/mL at 25 °C | ||
| Melting point | −4 °C (25 °F; 269 K) racemate | ||
| Boiling point | 99 to 103 °C (210 to 217 °F; 372 to 376 K) 10 mmHg | ||
| 66 g/L (20 °C) | |||
| Vapor pressure | 0.0533 Torr | ||
| Acidity (pKa) | 2.95±0.10. Most Acidic Temp: 25 °C | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
Causes severe skin burns and eye damage. Causes serious eye damage. Harmful if swallowed. | ||
| GHS labelling: | |||
  | |||
| Danger | |||
| H302, H314 | |||
| P260, P264, P270, P280, P301+P312, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P330, P363, P405, P501 | |||
| Flash point | > 112 °C (234 °F; 385 K) | ||
| Related compounds | |||
Other anions |
2-Bromobutyride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
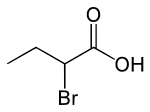
2-Bromobutyric acid is the organic compound with the molecular formula CH3CH2CH(Br)CO2H. It is a colorless liquid.
The 2-position is stereogenic, so the compound is chiral. Optical resolution can be effected using strychnine.[1]
2-Bromobutyric acid is used as a building block chemical, such as in the preparation of Levetiracetam, an anticonvulsant medication.[2]
Production
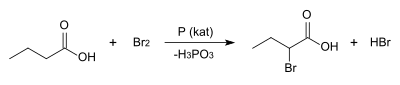
(±)-2-Bromobuyric acid may be prepared by the acid-catalyzed Hell–Volhard–Zelinsky reaction, where butyric acid is treated with elemental bromine. It is one of many compounds that arise adventiously from the use of bromine as an antiseptic.[3]
References
- ↑ Ahlberg, R. (1932). "Über die α-Brom-n-buttersäure. (II. Mitteilung). Die Zerlegung der racem-Säure". Journal für Praktische Chemie. 135 (11–12): 335–344. doi:10.1002/prac.19321351103.
- ↑ "Levetiracetam - PubMed Health". Archived from the original on 2012-11-30. Retrieved 2017-11-01.
- ↑ Krasner, Stuart W.; Weinberg, Howard S.; Richardson, Susan D.; Pastor, Salvador J.; Chinn, Russell; Sclimenti, Michael J.; Onstad, Gretchen D.; Thruston, Alfred D. (2006). "Occurrence of a New Generation of Disinfection Byproducts". Environmental Science & Technology. 40 (23): 7175–7185. doi:10.1021/es060353j. PMID 17180964.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.