 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylpropan-1-amine | |
| Other names
(2-Methylpropyl)amine | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| 385626 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.042 |
| EC Number |
|
| 81862 | |
| KEGG | |
| MeSH | isobutylamine |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1214 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H11N | |
| Molar mass | 73.139 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Fishy, ammoniacal |
| Density | 736 mg mL−1 |
| Melting point | −86.6 °C; −124.0 °F; 186.5 K |
| Boiling point | 67 to 69 °C; 152 to 156 °F; 340 to 342 K |
| Miscible | |
| -59.8·10−6 cm3/mol | |
Refractive index (nD) |
1.397 |
| Viscosity | 500 μPa s (at 20 °C) |
| Thermochemistry | |
Heat capacity (C) |
194 J K−1 mol−1 |
Std enthalpy of formation (ΔfH⦵298) |
−133.0–−132.0 kJ mol−1 |
Std enthalpy of combustion (ΔcH⦵298) |
−3.0139–−3.0131 MJ mol−1 |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H225, H301, H314 | |
| P210, P280, P301+P310, P305+P351+P338, P310 | |
| Flash point | −9 °C (16 °F; 264 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
224 mg kg−1 (oral, rat) |
| Related compounds | |
Related alkanamines |
|
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
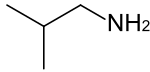
Isobutylamine is an organic chemical compound (specifically, an amine) with the formula (CH3)2CHCH2NH2, and occurs as a colorless liquid.[1][2] Isobutylamine is one of the four isomeric amines of butane, the others being n-butylamine, sec-butylamine and tert-butylamine. It is the decarboxylated form of the amino acid valine, and the product of the metabolism thereof by the enzyme valine decarboxylase.
Isobutylamine is an odorant binding to TAAR3 in mice and can trigger sexual behaviour in male mice dependent on the cluster of TAAR2 through TAAR9.[3]
References
- ↑ Isobutylamine chemicalbook.com
- ↑ Isobutylamine Chemblink.com
- ↑ Harmeier A, Meyer CA, Staempfli A, Casagrande F, Petrinovic MM, Zhang YP, Künnecke B, Iglesias A, Höner OP, Hoener MC (2018). "How Female Mice Attract Males: A Urinary Volatile Amine Activates a Trace Amine-Associated Receptor That Induces Male Sexual Interest". Frontiers in Pharmacology. 9: 924. doi:10.3389/fphar.2018.00924. PMC 6104183. PMID 30158871.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.