 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.163.612 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C27H46O2 | |
| Molar mass | 402.7 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
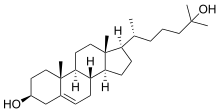
25-Hydroxycholesterol is a derivative of cholesterol, which plays a role in various biological processes in humans and other species. It is involved in cholesterol metabolism, antivirus process, inflammatory and immune response, and survival signaling pathway. 25-hydroxycholesterol is biosynthesized from cholesterol by adding a hydroxyl group at the position 25-carbon of a steroid nucleus. This reaction is catalyzed by cholesterol 25-hydroxylase, a family of enzymes that use oxygen and a di-iron cofactor to catalyze hydroxylation reaction.[1][2]
The CYP3A4 enzyme, a member of the cytochrome P450 family, can catalyze the oxidation of 25-hydroxycholesterol to 7α,25-dihydroxycholesterol, whereas 25-hydroxycholesterol can inhibit CYP4F2 mRNA expression, so that members of the cytochrome P450 family are also involved in the metabolism of 25-hydroxycholesterol besides cholesterol 25-hydroxylase.[3]
25-hydroxycholesterol has been found in various organisms such as mice, rats, rabbits, and cows. As of 2023, its presence in other species has not been extensively studied.[2][1]
See also
- cholesterol – sterol biosynthesized by all animal cells;
- cholesterol 25-hydroxylase – class of enzymes;
- 27-hydroxycholesterol – chemical compound.
References
- 1 2 Zhang J, Zhu Y, Wang X, Wang J (2023). "25-hydroxycholesterol: an integrator of antiviral ability and signaling". Front Immunol. 14: 1268104. doi:10.3389/fimmu.2023.1268104. PMC 10533924. PMID 37781400.
- 1 2 Cao Q, Liu Z, Xiong Y, Zhong Z, Ye Q (2020). "Multiple Roles of 25-Hydroxycholesterol in Lipid Metabolism, Antivirus Process, Inflammatory Response, and Cell Survival". Oxid Med Cell Longev. 2020: 8893305. doi:10.1155/2020/8893305. PMC 7695496. PMID 33274010.
- ↑ Hsu MH, Savas U, Griffin KJ, Johnson EF (February 2007). "Regulation of human cytochrome P450 4F2 expression by sterol regulatory element-binding protein and lovastatin". J Biol Chem. 282 (8): 5225–36. doi:10.1074/jbc.M608176200. PMID 17142457.
Further reading
- Zu S, Deng Y, Zhou C, Li J, Li L, Chen Q, Li X, Zhao H, Gold S, He J, Li X, Zhang C, Yang H, Cheng G, Qin C (November 2020). "25-Hydroxycholesterol is a potent SARS-CoV-2 inhibitor". Cell Research. 30 (11): 1043–1045. doi:10.1038/s41422-020-00398-1. ISSN 1748-7838. PMC 7431750. PMID 32811977. S2CID 221146053.