 | |
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
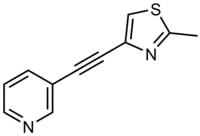
| Formula | C11H8N2S |
| Molar mass | 200.26 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
3-((2-Methyl-4-thiazolyl)ethynyl)pyridine (MTEP) is a research drug that was developed by Merck & Co. as a selective allosteric antagonist of the metabotropic glutamate receptor subtype mGluR5. Identified through structure-activity relationship studies on an older mGluR5 antagonist MPEP,[1] MTEP has subsequently itself acted as a lead compound for newer and even more improved drugs.[2][3]
MTEP is both more potent and more selective than MPEP as a mGluR5 antagonist,[4] and produces similar neuroprotective,[5][6][7] antidepressant,[8][9][10][11] analgesic,[12][13] and anxiolytic effects but with either similar or higher efficacy depending on the test used.[14][15][16][17]
MTEP also has similar efficacy to MPEP in reducing the symptoms of morphine withdrawal,[18][19][20] and has anti-addictive effects in a variety of animal models, both reducing ethanol self-administration,[21][22][23][24] and also decreasing the addictive effects of nicotine, cocaine and methamphetamine.[25][26][27][28][29]
See also
References
- ↑ Cosford ND, Tehrani L, Roppe J, Schweiger E, Smith ND, Anderson J, et al. (January 2003). "3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: a potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity". Journal of Medicinal Chemistry. 46 (2): 204–206. doi:10.1021/jm025570j. PMID 12519057.
- ↑ Iso Y, Grajkowska E, Wroblewski JT, Davis J, Goeders NE, Johnson KM, et al. (February 2006). "Synthesis and structure-activity relationships of 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine analogues as potent, noncompetitive metabotropic glutamate receptor subtype 5 antagonists; search for cocaine medications". Journal of Medicinal Chemistry. 49 (3): 1080–1100. doi:10.1021/jm050570f. PMID 16451073.
- ↑ Kulkarni SS, Newman AH (June 2007). "Discovery of heterobicyclic templates for novel metabotropic glutamate receptor subtype 5 antagonists". Bioorganic & Medicinal Chemistry Letters. 17 (11): 2987–2991. doi:10.1016/j.bmcl.2007.03.066. PMC 1973162. PMID 17446071.
- ↑ Lea PM, Faden AI (2006). "Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP". CNS Drug Reviews. 12 (2): 149–166. doi:10.1111/j.1527-3458.2006.00149.x. PMC 6494124. PMID 16958988.
- ↑ Lea PM, Movsesyan VA, Faden AI (June 2005). "Neuroprotective activity of the mGluR5 antagonists MPEP and MTEP against acute excitotoxicity differs and does not reflect actions at mGluR5 receptors". British Journal of Pharmacology. 145 (4): 527–534. doi:10.1038/sj.bjp.0706219. PMC 1576169. PMID 15821750.
- ↑ Domin H, Kajta M, Smiałowska M (2006). "Neuroprotective effects of MTEP, a selective mGluR5 antagonists and neuropeptide Y on the kainate-induced toxicity in primary neuronal cultures". Pharmacological Reports. 58 (6): 846–858. PMID 17220542.
- ↑ Szydlowska K, Kaminska B, Baude A, Parsons CG, Danysz W (January 2007). "Neuroprotective activity of selective mGlu1 and mGlu5 antagonists in vitro and in vivo". European Journal of Pharmacology. 554 (1): 18–29. doi:10.1016/j.ejphar.2006.09.061. PMID 17109843.
- ↑ Pałucha A, Brański P, Szewczyk B, Wierońska JM, Kłak K, Pilc A (August 2005). "Potential antidepressant-like effect of MTEP, a potent and highly selective mGluR5 antagonist". Pharmacology, Biochemistry, and Behavior. 81 (4): 901–906. doi:10.1016/j.pbb.2005.06.015. PMID 16040106. S2CID 42920507.
- ↑ Molina-Hernández M, Tellez-Alcántara NP, Pérez-García J, Olivera-Lopez JI, Jaramillo MT (August 2006). "Antidepressant-like and anxiolytic-like actions of the mGlu5 receptor antagonist MTEP, microinjected into lateral septal nuclei of male Wistar rats". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 30 (6): 1129–1135. doi:10.1016/j.pnpbp.2006.04.022. PMID 16759778. S2CID 45937198.
- ↑ Li X, Need AB, Baez M, Witkin JM (October 2006). "Metabotropic glutamate 5 receptor antagonism is associated with antidepressant-like effects in mice". The Journal of Pharmacology and Experimental Therapeutics. 319 (1): 254–259. doi:10.1124/jpet.106.103143. PMID 16803860. S2CID 14632318.
- ↑ Belozertseva IV, Kos T, Popik P, Danysz W, Bespalov AY (February 2007). "Antidepressant-like effects of mGluR1 and mGluR5 antagonists in the rat forced swim and the mouse tail suspension tests". European Neuropsychopharmacology. 17 (3): 172–179. doi:10.1016/j.euroneuro.2006.03.002. PMID 16630709. S2CID 2420850.
- ↑ Zhu CZ, Wilson SG, Mikusa JP, Wismer CT, Gauvin DM, Lynch JJ, et al. (December 2004). "Assessing the role of metabotropic glutamate receptor 5 in multiple nociceptive modalities". European Journal of Pharmacology. 506 (2): 107–118. doi:10.1016/j.ejphar.2004.11.005. PMID 15588730.
- ↑ Varty GB, Grilli M, Forlani A, Fredduzzi S, Grzelak ME, Guthrie DH, et al. (April 2005). "The antinociceptive and anxiolytic-like effects of the metabotropic glutamate receptor 5 (mGluR5) antagonists, MPEP and MTEP, and the mGluR1 antagonist, LY456236, in rodents: a comparison of efficacy and side-effect profiles". Psychopharmacology. 179 (1): 207–217. doi:10.1007/s00213-005-2143-4. PMID 15682298. S2CID 21807900.
- ↑ Klodzinska A, Tatarczyńska E, Chojnacka-Wójcik E, Nowak G, Cosford ND, Pilc A (September 2004). "Anxiolytic-like effects of MTEP, a potent and selective mGlu5 receptor agonist does not involve GABA(A) signaling". Neuropharmacology. 47 (3): 342–350. doi:10.1016/j.neuropharm.2004.04.013. PMID 15275823. S2CID 54432014.
- ↑ Busse CS, Brodkin J, Tattersall D, Anderson JJ, Warren N, Tehrani L, et al. (November 2004). "The behavioral profile of the potent and selective mGlu5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) in rodent models of anxiety". Neuropsychopharmacology. 29 (11): 1971–1979. doi:10.1038/sj.npp.1300540. PMID 15305166.
- ↑ Pietraszek M, Sukhanov I, Maciejak P, Szyndler J, Gravius A, Wisłowska A, et al. (May 2005). "Anxiolytic-like effects of mGlu1 and mGlu5 receptor antagonists in rats". European Journal of Pharmacology. 514 (1): 25–34. doi:10.1016/j.ejphar.2005.03.028. PMID 15878321.
- ↑ Stachowicz K, Gołembiowska K, Sowa M, Nowak G, Chojnacka-Wójcik E, Pilc A (November 2007). "Anxiolytic-like action of MTEP expressed in the conflict drinking Vogel test in rats is serotonin dependent". Neuropharmacology. 53 (6): 741–748. doi:10.1016/j.neuropharm.2007.08.002. PMID 17870136. S2CID 24833690.
- ↑ Pałucha A, Brański P, Pilc A (2004). "Selective mGlu5 receptor antagonist MTEP attenuates naloxone-induced morphine withdrawal symptoms" (PDF). Polish Journal of Pharmacology. 56 (6): 863–866. PMID 15662102.
- ↑ Rasmussen K, Martin H, Berger JE, Seager MA (February 2005). "The mGlu5 receptor antagonists MPEP and MTEP attenuate behavioral signs of morphine withdrawal and morphine-withdrawal-induced activation of locus coeruleus neurons in rats". Neuropharmacology. 48 (2): 173–180. doi:10.1016/j.neuropharm.2004.09.010. PMID 15695156. S2CID 13552709.
- ↑ Kotlinska J, Bochenski M (March 2007). "Comparison of the effects of mGluR1 and mGluR5 antagonists on the expression of behavioral sensitization to the locomotor effect of morphine and the morphine withdrawal jumping in mice". European Journal of Pharmacology. 558 (1–3): 113–118. doi:10.1016/j.ejphar.2006.11.067. PMID 17222405.
- ↑ Cowen MS, Djouma E, Lawrence AJ (November 2005). "The metabotropic glutamate 5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine reduces ethanol self-administration in multiple strains of alcohol-preferring rats and regulates olfactory glutamatergic systems". The Journal of Pharmacology and Experimental Therapeutics. 315 (2): 590–600. doi:10.1124/jpet.105.090449. PMID 16014750. S2CID 11501029.
- ↑ Cowen MS, Krstew E, Lawrence AJ (January 2007). "Assessing appetitive and consummatory phases of ethanol self-administration in C57BL/6J mice under operant conditions: regulation by mGlu5 receptor antagonism". Psychopharmacology. 190 (1): 21–29. doi:10.1007/s00213-006-0583-0. PMID 17096086. S2CID 19977179.
- ↑ Adams CL, Cowen MS, Short JL, Lawrence AJ (March 2008). "Combined antagonism of glutamate mGlu5 and adenosine A2A receptors interact to regulate alcohol-seeking in rats". The International Journal of Neuropsychopharmacology. 11 (2): 229–241. doi:10.1017/S1461145707007845. hdl:11343/32923. PMID 17517168.
- ↑ Kotlinska J, Bochenski M (November 2008). "The influence of various glutamate receptors antagonists on anxiety-like effect of ethanol withdrawal in a plus-maze test in rats". European Journal of Pharmacology. 598 (1–3): 57–63. doi:10.1016/j.ejphar.2008.09.026. PMID 18838071.
- ↑ Dravolina OA, Danysz W, Bespalov AY (September 2006). "Effects of group I metabotropic glutamate receptor antagonists on the behavioral sensitization to motor effects of cocaine in rats". Psychopharmacology. 187 (4): 397–404. doi:10.1007/s00213-006-0440-1. PMID 16896963. S2CID 21231365.
- ↑ Palmatier MI, Liu X, Donny EC, Caggiula AR, Sved AF (August 2008). "Metabotropic glutamate 5 receptor (mGluR5) antagonists decrease nicotine seeking, but do not affect the reinforcement enhancing effects of nicotine". Neuropsychopharmacology. 33 (9): 2139–2147. doi:10.1038/sj.npp.1301623. PMC 2812904. PMID 18046312.
- ↑ Gass JT, Osborne MP, Watson NL, Brown JL, Olive MF (March 2009). "mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats". Neuropsychopharmacology. 34 (4): 820–833. doi:10.1038/npp.2008.140. PMC 2669746. PMID 18800068.
- ↑ Osborne MP, Olive MF (October 2008). "A role for mGluR5 receptors in intravenous methamphetamine self-administration". Annals of the New York Academy of Sciences. 1139 (1): 206–211. Bibcode:2008NYASA1139..206O. doi:10.1196/annals.1432.034. PMID 18991866. S2CID 207012906.
- ↑ Martin-Fardon R, Baptista MA, Dayas CV, Weiss F (June 2009). "Dissociation of the effects of MTEP [3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine] on conditioned reinstatement and reinforcement: comparison between cocaine and a conventional reinforcer". The Journal of Pharmacology and Experimental Therapeutics. 329 (3): 1084–1090. doi:10.1124/jpet.109.151357. PMC 2683783. PMID 19258516.