 | |
| Names | |
|---|---|
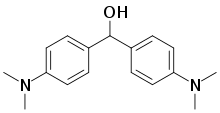
| Preferred IUPAC name
Bis[4-(dimethylamino)phenyl]methanol | |
| Other names
Michler's hydrol; 4,4′-Bisdimethylaminodiphenylcarbinol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.003.941 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C17H22N2O | |
| Molar mass | 270.376 g·mol−1 |
| Appearance | White solid |
| Melting point | 98–100 °C (208–212 °F; 371–373 K) |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
4,4′-Bis(dimethylamino)benzhydrol is an organic compound with the formula (Me2NC6H4)2CH(OH), where Me is methyl. It is a white solid that is soluble is a variety of organic solvents. The compound is notable as the reduced derivative of Michler's ketone. It is a precursor to triarylmethane dyes.[1]
References
- ↑ Muthyala, Ramaiah; Katritzky, Alan R.; Lan, Xiangfu (1994). "A synthetic study on the preparation of triarylmethanes". Dyes and Pigments. 25 (4): 303–324. doi:10.1016/0143-7208(94)87017-9.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.