| 5′-nucleotidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Human ecto-5'-nucleotidase (CD73): crystal form I (open) in complex with adenosine[1] | |||||||||
| Identifiers | |||||||||
| EC no. | 3.1.3.5 | ||||||||
| CAS no. | 9027-73-0 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
5′-Nucleotidase (EC 3.1.3.5) is an enzyme which catalyzes the phosphorylytic cleavage of 5′-nucleotides.[2] Although originally found in snake venom,[3] the activity of 5'nucleotidase has been described for bacteria and plant cells, and is widely distributed in vertebrate tissue.[4] In mammalian cells the enzyme is predominantly located in the plasma membrane and its primary role is in the conversion of extracellular nucleotides (e.g. 5'-AMP), which are generally impermeable, to the corresponding nucleoside (e.g. adenosine) which can readily enter most cells.[5] Consequently, the enzyme plays a key role in the metabolism of nucleotides.
The enzyme has a wide substrate specificity for nucleotides and has been shown to hydrolyze 5'nucleotides rapidly, ribose-5-phosphate slowly, and other phosphate esters extremely slowly (if at all).[6]
The enzyme catalyses the following reaction:
- a 5′-nucleotide + H2O ⇌ a nucleoside + phosphate
The 5′-nucleotidase-catalyzed reaction of an AMP nucleotide to adenosine nucleoside is shown below:

Nomenclature
- Accepted Name: 5′-nucleotidase
- Systematic Name: 5′-ribonucleotide phosphohydrolase
- Synonyms: uridine 5′-nucleotidase, 5′-adenylic phosphatase, adenosine 5'-phosphatase, AMP phosphatase, adenosine monophosphatase, 5′-mononucleotidase, AMPase, UMPase, 'snake venom 5'-nucleotidase, thimidine monophosphate nucleotidase, 5′-AMPase, 5′-AMP nucleotidase, AMP phosphohydrolase, IMP 5′-nucleotidase).[7][8][9]
Structure

Active site
Studies of the soluble form of human ecto-5′-nucleotidase, without a GPI anchor, have shown that the C-terminal domain holds the substrate-binding pocket, and that the aromatic purine motif of the substrate is stacked between two phenylalanine residues.[10] Furthermore, a catalytic mechanism has been proposed involving an in-line nucleophilic attack by a hydroxyl moiety that is coordinated by zinc on the substrate phosphorus, with the nucleoside acting as a leaving group.[10]
Membrane-bound and soluble forms
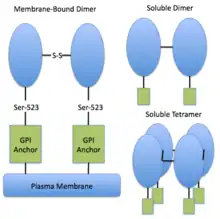
Studies of mammalian 5′-nucleotidases have shown that there exist at least four different forms of the 5′-nucleotidase enzyme: one membrane-bound form and three soluble forms.[4] The membrane-bound form is anchored to the plasma membrane via GPI at its C-terminus.[4] One of the soluble forms appears to be derived from the GPI-anchored ex-5'-nucleotidase and has an extracellular location.[4] The two cytosolic forms of the enzyme have similar characteristics, but can be differentiated on the basis of their preferential affinities for nucleotide substrates.[4] The GPI-anchored form exists as a dimer, with the two subunits linked via a disulfide bridge. The soluble forms can exist as dimers or tetramers. Generally at least 50% of the enzyme is found in the surface-bound form.[4]
Medical relevance
5' Nucleotidase blood test
The concentration of 5’nucleotidase protein in the blood is often used as a liver function test in individuals that show signs of liver problems. The combined assays of serum 5'nucleotisase and alkaline phosphatase (AP) activities are extremely helpful in differential diagnosis since serum 5'nucleotidase activity is increased in obstructive hepatobiliary disorders, but not in osseous disorders, whereas serum AP activity is generally increased in both categories of diseases.[11] In other words, the test is used to determine if elevated protein levels are due to skeletal damage or liver damage.[12] Normal levels of 5’nucleotidase are 2-17 units per liter.[13] Elevated levels may indicate cholestasis, destruction of liver cells, hepatitis (liver inflammation), liver ischemia, a liver tumor, or use of liver-damaging drugs. Pregnancy and certain medications (acetaminophen, halothane, isoniazid, methyldopa, nitrofurantoin) may interfere with the test.[12][13]
The test may also be referred to as 5'NT Levels Blood Test, CDF73 Levels Blood Test, and Ecto-5'-Nucleotidase Levels Blood Test.[12]
Lymphocyte 5'nucleotidase in immunological disorders
Ecto-5'Nucleotidase is considered a maturation marker for T cells and B cells. This is due to the fact that the enzyme activity is approximately 10-times higher for peripheral T cells than thymocytes, 5-6 times higher in adult peripheral B cells than fetal B cells, and largely absent in non-T cell and non-B cell lymphocytes.[5][14] In immunodeficiency diseases with arrested lymphocyte maturation, ex-5'nucleatidase activity is generally low.[5] Such diseases include severe combined immuno-deficiency, Wiskott–Aldrich syndrome, congenital X-linked agammaglobulinemia, selective IgA deficiency and acquired immune deficiency syndrome (AIDS).[5][15][16][17]
Relation to lead poisoning
Numerous studies have shown that erythrocyte pyrimidine 5′-nucleotidase activity is significantly lowered in patients with lead poisoning, and that pyrimidine 5′-nucleotidase activity can be used as an index of lead poisoning.[18] It is believed that lead induced deficiency of the enzyme in maturing erythroid cells is responsible for basophilic stippling and hemolysis in a manner analogous to the pathogenesis of the hereditary enzyme deficiency syndrome. The mechanism of inhibition of 5'nucleotidase in lead poisoning may contribute to the hemolytic syndromes that occur in patients with acute lead poisoning.[5] Since erythrocyte pyrimidine 5'NT activity is inhibited in vitro by various metals (e.g., copper, zinc, cadmium, lead, mercury, and tin), it is likely that inhibition of pyrimidine 5'nucleotidase may contribute to the hemolytic syndromes that occur in patients with acute poisoning by these metals.[5][19][20]
Cytosolic 5-nucleotidase II superactivity
Cytosolic 5-nucleotidase II superactivity has been associated with autism spectrum disorder via a disorder of carnitine biosynthesis.[21]
References
- ↑ Knapp K, Zebisch M, Pippel J, El-Tayeb A, Müller CE, Sträter N (December 2012). "Crystal structure of the human ecto-5'-nucleotidase (CD73): insights into the regulation of purinergic signaling". Structure. 20 (12): 2161–73. doi:10.1016/j.str.2012.10.001. PMID 23142347.
- ↑ Fleit H, Conklyn M, Stebbins RD, Silber R (December 1975). "Function of 5′-nucleotidase in the uptake of adenosine from AMP by human lymphocytes" (PDF). The Journal of Biological Chemistry. 250 (23): 8889–92. doi:10.1016/S0021-9258(19)40668-6. PMID 1194267.
- ↑ Reis, Julian (1934). "Nucleotidase and its relation to the deamination of nucleotides in the heart and the muscles". Bulletin de la Société de Chimie Biologique. 16: 385–399.
- 1 2 3 4 5 6 Sidorov VP (1975). "[Factors affecting the frequency of exploratory thoracotomies in lung cancer]". Grudnaia Khirurgiia (2): 84–87. PMID 1132794.
- 1 2 3 4 5 6 Sunderman FW (1990). "The clinical biochemistry of 5'-nucleotidase" (PDF). Annals of Clinical and Laboratory Science. 20 (2): 123–39. PMID 2183704.
- ↑ Koshland DE, Springhorn SS (July 1956). "Mechanism of action of 5'-nucleotidase". The Journal of Biological Chemistry. 221 (1): 469–76. doi:10.1016/S0021-9258(18)65265-2. PMID 13345835.
- ↑ Gulland JM, Jackson EM (March 1938). "5-Nucleotidase". The Biochemical Journal. 32 (3): 597–601. doi:10.1042/bj0320597. PMC 1264072. PMID 16746659.
- ↑ Heppel LA, Hilmore RJ (February 1951). "Purification and properties of 5-nucleotidase". The Journal of Biological Chemistry. 188 (2): 665–76. doi:10.1016/S0021-9258(19)77739-4. PMID 14824154.
- ↑ Segal HL, Brenner BM (February 1960). "5′-Nucleotidase of rat liver microsomes". The Journal of Biological Chemistry. 235 (2): 471–4. doi:10.1016/S0021-9258(18)69548-1. PMID 14444527.
- 1 2 3 Heuts DP, Weissenborn MJ, Olkhov RV, Shaw AM, Gummadova J, Levy C, Scrutton NS (November 2012). "Crystal structure of a soluble form of human CD73 with ecto-5′-nucleotidase activity". ChemBioChem. 13 (16): 2384–91. doi:10.1002/cbic.201200426. PMID 22997138. S2CID 44660514.
- ↑ Dixon TF, Purdom M (November 1954). "Serum 5-nucleotidase". Journal of Clinical Pathology. 7 (4): 341–3. doi:10.1136/jcp.7.4.341. PMC 1023849. PMID 13286361.
- 1 2 3 "5'Nucleotidase Blood Test". DoveMed. Retrieved 28 February 2016.
- 1 2 Greco, Frank. "5'nucleotidase". MedlinePlus. U.S. National Library of Medicine. Retrieved 28 February 2016.
- ↑ Edwards NL, Gelfand EW, Burk L, Dosch HM, Fox IH (July 1979). "Distribution of 5'-nucleotidase in human lymphoid tissues". Proceedings of the National Academy of Sciences of the United States of America. 76 (7): 3474–6. Bibcode:1979PNAS...76.3474E. doi:10.1073/pnas.76.7.3474. PMC 383848. PMID 315065.
- ↑ Salazar-Gonzalez JF, Moody DJ, Giorgi JV, Martinez-Maza O, Mitsuyasu RT, Fahey JL (September 1985). "Reduced ecto-5'-nucleotidase activity and enhanced OKT10 and HLA-DR expression on CD8 (T suppressor/cytotoxic) lymphocytes in the acquired immune deficiency syndrome: evidence of CD8 cell immaturity". Journal of Immunology. 135 (3): 1778–85. PMID 2991373.
- ↑ Webster AD, North M, Allsop J, Asherson GL, Watts RW (March 1978). "Purine metabolism in lymphocytes from patients with primary hypogammaglobulinaemia". Clinical and Experimental Immunology. 31 (3): 456–63. PMC 1541241. PMID 207476.
- ↑ Boss GR, Thompson LF, O'Connor RD, Ziering RW, Seegmiller JE (April 1981). "Ecto-5'-nucleotidase deficiency: association with adenosine deaminase deficiency and nonassociation with deoxyadenosine toxicity". Clinical Immunology and Immunopathology. 19 (1): 1–7. doi:10.1016/0090-1229(81)90042-8. PMID 6260402.
- ↑ Paglia DE, Valentine WN, Dahlgren JG (November 1975). "Effects of low-level lead exposure on pyrimidine 5′-nucleotidase and other erythrocyte enzymes. Possible role of pyrimidine 5′-nucleotidase in the pathogenesis of lead-induced anemia". The Journal of Clinical Investigation. 56 (5): 1164–9. doi:10.1172/jci108192. PMC 301979. PMID 1184742.
- ↑ Cook L, Kubitschek C, Stohs S, Angle C (June 1988). "Erythrocyte pyrimidine 5'-nucleotidase and deoxynucleotidase isozymes: metallosensitivity and kinetics". Drug and Chemical Toxicology. 11 (2): 195–213. doi:10.3109/01480548808998222. PMID 2841083.
- ↑ Mohammed-Brahim B, Buchet JP, Bernard A, Lauwerys R (February 1984). "In vitro effects of lead, mercury and cadmium on the enzymic activity of red-blood cell pyrimidine 5′-nucleotidase". Toxicology Letters. 20 (2): 195–9. doi:10.1016/0378-4274(84)90147-4. PMID 6320500.
- ↑ Hyman, Susan L.; Levy, Susan E.; Myers, Scott M.; COUNCIL ON CHILDREN WITH DISABILITIES, SECTION ON DEVELOPMENTAL AND BEHAVIORAL PEDIATRICS; Kuo, Dennis Z.; Apkon, Susan; Davidson, Lynn F.; Ellerbeck, Kathryn A.; Foster, Jessica E.A.; Noritz, Garey H.; Leppert, Mary O’Connor (2020-01-01). "Identification, Evaluation, and Management of Children With Autism Spectrum Disorder". Pediatrics. 145 (1): e20193447. doi:10.1542/peds.2019-3447. ISSN 0031-4005. PMID 31843864. S2CID 209390456.
External links
- 5'-nucleotidase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)