 | |
| Names | |
|---|---|
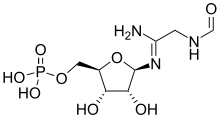
| IUPAC name
[(2R,3S,4R,5R)-5-[(1-Amino-2-formamidoethylidene)amino]-3,4-dihydroxyoxolan-2-yl]methyl dihydrogen phosphate | |
| Other names
FGAM | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C8H16N3O8P | |
| Molar mass | 313.20 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
5′-Phosphoribosylformylglycinamidine (or FGAM) is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, and hence is a building block for DNA and RNA.[1][2] The vitamins thiamine[3][4] and cobalamin[5] also contain fragments derived from FGAM.[6]
The compound is biosynthesized from phosphoribosyl-N-formylglycineamide (FGAR) which is converted to an amidine by the action of phosphoribosylformylglycinamidine synthase (EC 6.3.5.3), transferring an amino group from glutamine in a reaction that also requires ATP:
- FGAR + ATP + glutamine + H2O → FGAM + ADP + glutamate + Pi
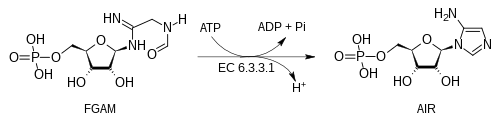
The biosynthesis pathway next converts FGAM to 5-aminoimidazole ribotide (AIR) by the action of AIR synthetase (EC 6.3.3.1) which uses ATP to activate the terminal carbonyl group to attack by the nitrogen atom at the anomeric center:
- FGAM + ATP → AIR + ADP + Pi + H+
See also
References
- ↑ R. Caspi (2009-01-13). "Pathway: 5-aminoimidazole ribonucleotide biosynthesis I". MetaCyc Metabolic Pathway Database. Retrieved 2022-02-02.
- ↑ Gupta, Rani; Gupta, Namita (2021). "Nucleotide Biosynthesis and Regulation". Fundamentals of Bacterial Physiology and Metabolism. pp. 525–554. doi:10.1007/978-981-16-0723-3_19. ISBN 978-981-16-0722-6. S2CID 234897784.
- ↑ R. Caspi (2011-09-14). "Pathway: superpathway of thiamine diphosphate biosynthesis I". MetaCyc Metabolic Pathway Database. Retrieved 2022-02-01.
- ↑ Chatterjee, Abhishek; Hazra, Amrita B.; Abdelwahed, Sameh; Hilmey, David G.; Begley, Tadhg P. (2010). "A "Radical Dance" in Thiamin Biosynthesis: Mechanistic Analysis of the Bacterial Hydroxymethylpyrimidine Phosphate Synthase". Angewandte Chemie International Edition. 49 (46): 8653–8656. doi:10.1002/anie.201003419. PMC 3147014. PMID 20886485.
- ↑ R. Caspi (2019-09-23). "Pathway: 5-hydroxybenzimidazole biosynthesis (anaerobic)". MetaCyc Metabolic Pathway Database. Retrieved 2022-02-10.
- ↑ Mehta, Angad P.; Abdelwahed, Sameh H.; Fenwick, Michael K.; Hazra, Amrita B.; Taga, Michiko E.; Zhang, Yang; Ealick, Steven E.; Begley, Tadhg P. (2015). "Anaerobic 5-Hydroxybenzimidazole Formation from Aminoimidazole Ribotide: An Unanticipated Intersection of Thiamin and Vitamin B12 Biosynthesis". Journal of the American Chemical Society. 137 (33): 10444–10447. doi:10.1021/jacs.5b03576. PMC 4753784. PMID 26237670.