| ALDOC | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | ALDOC, ALDC, Aldolase C, aldolase, fructose-bisphosphate C | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 103870 MGI: 101863 HomoloGene: 21073 GeneCards: ALDOC | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Aldolase C, fructose-bisphosphate (ALDOC, or ALDC), is an enzyme that, in humans, is encoded by the ALDOC gene on chromosome 17. This gene encodes a member of the class I fructose-bisphosphate aldolase gene family. Expressed specifically in the hippocampus and Purkinje cells of the brain, the encoded protein is a glycolytic enzyme that catalyzes the reversible aldol cleavage of fructose 1,6-bisphosphate and fructose-1-phosphate to dihydroxyacetone phosphate and either glyceraldehyde 3-phosphate or glyceraldehyde, respectively.[provided by RefSeq, Jul 2008][5][6]
Structure
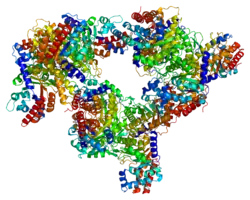
ALDOC is one of the three aldolase isozymes (A, B, and C), encoded by three different genes.[7][8] The amino acid sequence of ALDOC is highly similar to those of the other isozymes, sharing a 68% identity with ALDOB and 78% identity with ALDOA. In particular, the residues Asp33, Arg42, Lys107, Lys146, Glu187, Ser271, Arg303, and Lys229 are all conserved in the active sites of the three isozymes. This active site is located in the center of the homotetrameric αβ-barrel structure of these aldolases. However, several structural details set ALDOC apart. For instance, the Arg303 residue in ALDOC adopts an intermediate conformation between the liganded and unliganded structures observed in the other isozymes. Also, the C-terminal region between Glu332 and Lys71 forms a salt bridge with the barrel region that is absent in the A and B isoforms. Moreover, the electrostatic surface of ALDOC is more negatively charged, which may serve as an acidic binding site or as a docking site to accommodate the C-terminal conformations.[8] Four ALDOC-specific residues (N90, V92, R96 and D100) may be key for ALDOC-specific functions.[9]
Function
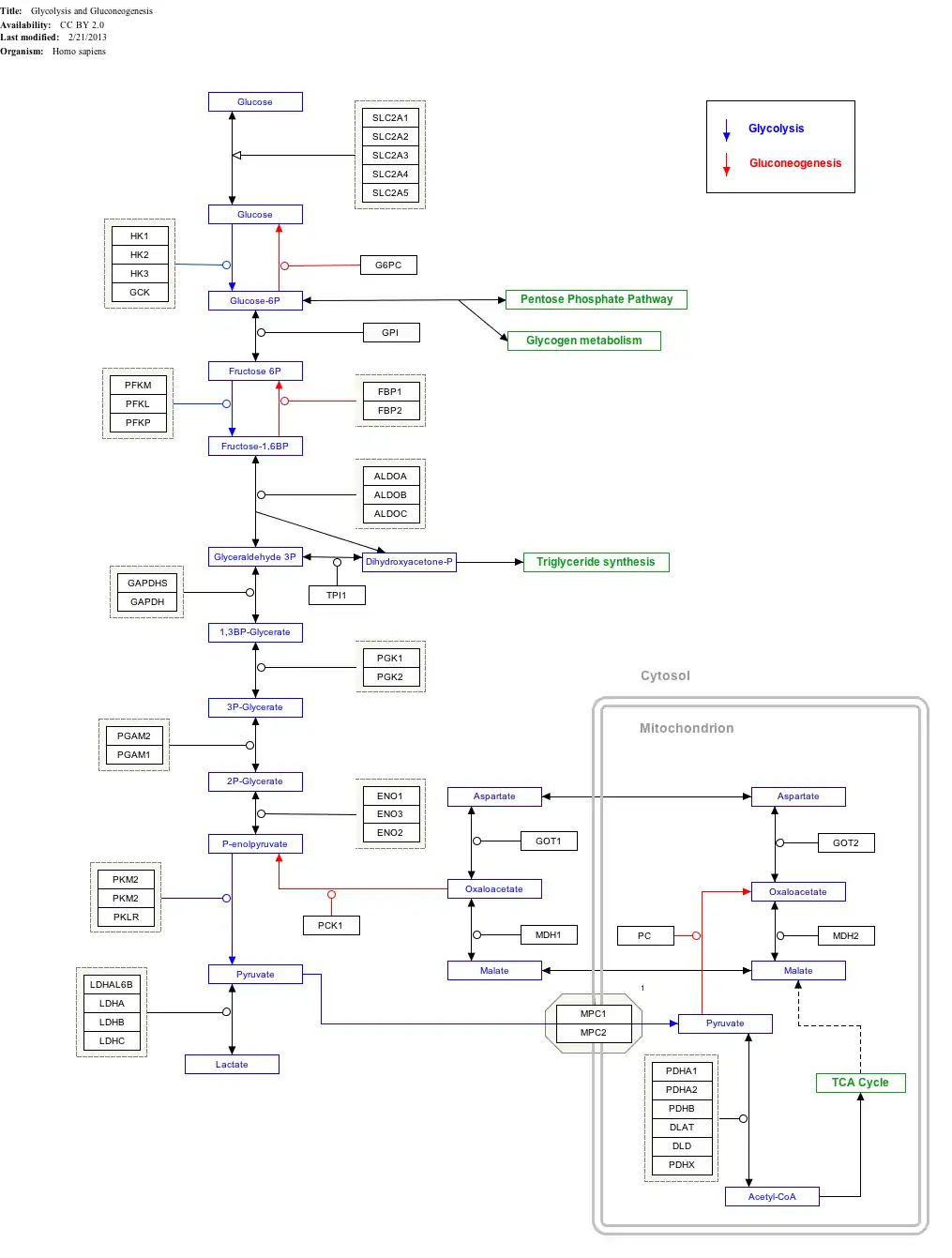
ALDOC is a key enzyme in the fourth step of glycolysis, as well as in the reverse pathway gluconeogenesis. It catalyzes the reversible conversion of fructose-1,6-bisphosphate to glyceraldehydes-3-phosphate (G3P), or glyceraldehyde, and dihydroxyacetone phosphate (DHAP) by aldol cleavage. As a result, it is a crucial player in ATP biosynthesis.[8][9] As an aldolase, ALDOC putatively also contributes to other "moonlighting" functions, though its exact involvements remain unclear.[8][9] For instance, it binds less tightly to the cytoskeleton than the other isozymes do, likely due to its more acidic pI.[8] In addition, ALDOC participates in the stress-response pathway for lung epithelial cell function during hypoxia and in the resistance of cerebellar Purkinje cells against excitotoxic insult.[10]
ALDOC is ubiquitously expressed in most tissues, though it is predominantly expressed in brain, smooth muscle, and neuronal tissue.[8][9][11][12] However, since the ALDOA isoform is co-expressed with ALDOC in the central nervous system (CS), it is suggested that ALDOC contributes to CNS function outside of glycolysis.[9] Moreover, its presence within other cell types, such as platelets and mast cells (MCs), may serve as a failsafe in the case that the other predominant aldolase isozymes become inactivated.[11] Within cells, it localizes to the cytoplasm.[12]
Clinical significance
This aldolase has been associated with cancer.[8]
ALDOC is found to be upregulated in the brains of schizophrenia (SCZ) patients.[13] Notably, while ALDOC is differentially expressed in the anterior cingulate cortex (ACC) of male SCZ patients, it displays no significant changes in female SCZ patients, indicating that different regulatory mechanisms may be involved in male versus female SCZ patients. It is likely that ALDOC is involved in SCZ through its role in glycolysis, which is a central biochemical pathway in SCZ.[14]
Furthermore, ALDOC is reported to undergo oxidation in brains affected by mild cognitive impairment (MCI) and Alzheimer's disease (AD). This oxidative modification inhibits ALDOC activity, causing the accumulation of fructose 1,6- bisphosphate and driving the reverse reaction, in the direction of gluconeogenesis rather than glycolysis, thus halting ATP production.[15]
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles.[§ 1]
- ↑ The interactive pathway map can be edited at WikiPathways: "GlycolysisGluconeogenesis_WP534".
See also
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000109107 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000017390 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Entrez Gene: ALDOC aldolase C, fructose-bisphosphate".
- ↑ Rocchi M, Vitale E, Covone A, Romeo G, Santamaria R, Buono P, Paolella G, Salvatore F (June 1989). "Assignment of human aldolase C gene to chromosome 17, region cen----q21.1". Human Genetics. 82 (3): 279–82. doi:10.1007/BF00291170. PMID 2731939. S2CID 7980799.
- ↑ Du S, Guan Z, Hao L, Song Y, Wang L, Gong L, Liu L, Qi X, Hou Z, Shao S (2014). "Fructose-bisphosphate aldolase a is a potential metastasis-associated marker of lung squamous cell carcinoma and promotes lung cell tumorigenesis and migration". PLOS ONE. 9 (1): e85804. Bibcode:2014PLoSO...985804D. doi:10.1371/journal.pone.0085804. PMC 3900443. PMID 24465716.
- 1 2 3 4 5 6 7 Arakaki TL, Pezza JA, Cronin MA, Hopkins CE, Zimmer DB, Tolan DR, Allen KN (December 2004). "Structure of human brain fructose 1,6-(bis)phosphate aldolase: linking isozyme structure with function". Protein Science. 13 (12): 3077–84. doi:10.1110/ps.04915904. PMC 2287316. PMID 15537755.
- 1 2 3 4 5 Langellotti S, Romano M, Guarnaccia C, Granata V, Orrù S, Zagari A, Baralle FE, Salvatore F (2014). "A novel anti-aldolase C antibody specifically interacts with residues 85-102 of the protein". mAbs. 6 (3): 708–17. doi:10.4161/mabs.28191. PMC 4011915. PMID 24525694.
- ↑ Slemmer JE, Haasdijk ED, Engel DC, Plesnila N, Weber JT (August 2007). "Aldolase C-positive cerebellar Purkinje cells are resistant to delayed death after cerebral trauma and AMPA-mediated excitotoxicity". The European Journal of Neuroscience. 26 (3): 649–56. doi:10.1111/j.1460-9568.2007.05708.x. PMID 17686042. S2CID 46706309.
- 1 2 Sekar Y, Moon TC, Slupsky CM, Befus AD (July 2010). "Protein tyrosine nitration of aldolase in mast cells: a plausible pathway in nitric oxide-mediated regulation of mast cell function". Journal of Immunology. 185 (1): 578–87. doi:10.4049/jimmunol.0902720. PMID 20511553.
- 1 2 Mamczur P, Gamian A, Kolodziej J, Dziegiel P, Rakus D (December 2013). "Nuclear localization of aldolase A correlates with cell proliferation". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1833 (12): 2812–2822. doi:10.1016/j.bbamcr.2013.07.013. PMID 23886627.
- ↑ Martins-de-Souza D, Gattaz WF, Schmitt A, Maccarrone G, Hunyadi-Gulyás E, Eberlin MN, Souza GH, Marangoni S, Novello JC, Turck CW, Dias-Neto E (July 2009). "Proteomic analysis of dorsolateral prefrontal cortex indicates the involvement of cytoskeleton, oligodendrocyte, energy metabolism and new potential markers in schizophrenia". Journal of Psychiatric Research. 43 (11): 978–86. doi:10.1016/j.jpsychires.2008.11.006. PMID 19110265.
- ↑ Martins-de-Souza D, Schmitt A, Röder R, Lebar M, Schneider-Axmann T, Falkai P, Turck CW (October 2010). "Sex-specific proteome differences in the anterior cingulate cortex of schizophrenia". Journal of Psychiatric Research. 44 (14): 989–91. doi:10.1016/j.jpsychires.2010.03.003. PMID 20381070.
- ↑ Sultana R, Perluigi M, Newman SF, Pierce WM, Cini C, Coccia R, Butterfield DA (March 2010). "Redox proteomic analysis of carbonylated brain proteins in mild cognitive impairment and early Alzheimer's disease". Antioxidants & Redox Signaling. 12 (3): 327–36. doi:10.1089/ars.2009.2810. PMC 2821142. PMID 19686046.
Further reading
- Buono P, Mancini FP, Izzo P, Salvatore F (September 1990). "Characterization of the transcription-initiation site and of the promoter region within the 5' flanking region of the human aldolase C gene". European Journal of Biochemistry. 192 (3): 805–11. doi:10.1111/j.1432-1033.1990.tb19294.x. PMID 2209624.
- Rocchi M, Vitale E, Covone A, Romeo G, Santamaria R, Buono P, Paolella G, Salvatore F (June 1989). "Assignment of human aldolase C gene to chromosome 17, region cen----q21.1". Human Genetics. 82 (3): 279–82. doi:10.1007/BF00291170. PMID 2731939. S2CID 7980799.
- Rottmann WH, Deselms KR, Niclas J, Camerato T, Holman PS, Green CJ, Tolan DR (February 1987). "The complete amino acid sequence of the human aldolase C isozyme derived from genomic clones". Biochimie. 69 (2): 137–45. doi:10.1016/0300-9084(87)90246-X. PMID 3105602.
- Buono P, Paolella G, Mancini FP, Izzo P, Salvatore F (May 1988). "The complete nucleotide sequence of the gene coding for the human aldolase C". Nucleic Acids Research. 16 (10): 4733. doi:10.1093/nar/16.10.4733. PMC 336672. PMID 3267224.
- Tolan DR, Niclas J, Bruce BD, Lebo RV (November 1987). "Evolutionary implications of the human aldolase-A, -B, -C, and -pseudogene chromosome locations". American Journal of Human Genetics. 41 (5): 907–24. PMC 1684339. PMID 3674018.
- Penhoet E, Rajkumar T, Rutter WJ (October 1966). "Multiple forms of fructose diphosphate aldolase in mammalian tissues". Proceedings of the National Academy of Sciences of the United States of America. 56 (4): 1275–82. Bibcode:1966PNAS...56.1275P. doi:10.1073/pnas.56.4.1275. PMC 220058. PMID 5230152.
- Andersson B, Wentland MA, Ricafrente JY, Liu W, Gibbs RA (April 1996). "A "double adaptor" method for improved shotgun library construction". Analytical Biochemistry. 236 (1): 107–13. doi:10.1006/abio.1996.0138. PMID 8619474.
- Yu W, Andersson B, Worley KC, Muzny DM, Ding Y, Liu W, Ricafrente JY, Wentland MA, Lennon G, Gibbs RA (April 1997). "Large-scale concatenation cDNA sequencing". Genome Research. 7 (4): 353–8. doi:10.1101/gr.7.4.353. PMC 139146. PMID 9110174.
- Kim JH, Lee S, Kim JH, Lee TG, Hirata M, Suh PG, Ryu SH (March 2002). "Phospholipase D2 directly interacts with aldolase via Its PH domain". Biochemistry. 41 (10): 3414–21. doi:10.1021/bi015700a. PMID 11876650.
- Arakaki TL, Pezza JA, Cronin MA, Hopkins CE, Zimmer DB, Tolan DR, Allen KN (December 2004). "Structure of human brain fructose 1,6-(bis)phosphate aldolase: linking isozyme structure with function". Protein Science. 13 (12): 3077–84. doi:10.1110/ps.04915904. PMC 2287316. PMID 15537755.
- Buono P, Barbieri O, Alfieri A, Rosica A, Astigiano S, Cantatore D, Mancini A, Fattoruso O, Salvatore F (December 2004). "Diverse human aldolase C gene promoter regions are required to direct specific LacZ expression in the hippocampus and Purkinje cells of transgenic mice". FEBS Letters. 578 (3): 337–44. doi:10.1016/j.febslet.2004.11.032. PMID 15589842. S2CID 41355547.
- Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, Grishin NV, White M, Yang XJ, Zhao Y (August 2006). "Substrate and functional diversity of lysine acetylation revealed by a proteomics survey". Molecular Cell. 23 (4): 607–18. doi:10.1016/j.molcel.2006.06.026. PMID 16916647.
External links
- Human ALDOC genome location and ALDOC gene details page in the UCSC Genome Browser.