 | |
| Names | |
|---|---|
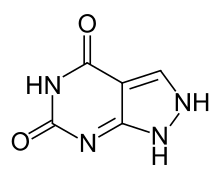
| Preferred IUPAC name
1H-pyrazolo[3,4-d]pyrimidine-4,6(2H,5H)-dione | |
| Other names
2,5-Dihydro-1H-pyrazolo[3,4-d]pyrimidine-4,6-dione Alloxanthine | |
| Identifiers | |
3D model (JSmol) |
|
| 139956 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.017.792 |
| EC Number |
|
| KEGG | |
| MeSH | Oxypurinol |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H4N4O2 | |
| Molar mass | 152.11086 |
| Appearance | white crystals |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Oxipurinol (INN, or oxypurinol USAN) is an inhibitor of xanthine oxidase.[1] It is an active metabolite of allopurinol and it is cleared renally.[2] In cases of renal disease, this metabolite will accumulate to toxic levels. By inhibiting xanthine oxidase, it reduces uric acid production. High serum uric acid levels may result in gout, kidney stones, and other medical conditions.
References
- ↑ Stocker, Sophie L; McLachlan, Andrew J; Savic, Radojka M; Kirkpatrick, Carl M; Graham, Garry G; Williams, Kenneth M; Day, Richard O (2012). "The pharmacokinetics of oxypurinol in people with gout". British Journal of Clinical Pharmacology. 74 (3): 477–489. doi:10.1111/j.1365-2125.2012.04207.x. PMC 3477349. PMID 22300439.
- ↑ Elion, Gertrude B; Yü, Ts'ai-Fan; Gutman, Alexander B; Hitchings, George H (1968). "Renal clearance of oxipurinol, the chief metabolite of allopurinol". The American Journal of Medicine. 45 (1): 69–77. doi:10.1016/0002-9343(68)90008-9. PMID 5658870.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.