 | |
| Names | |
|---|---|
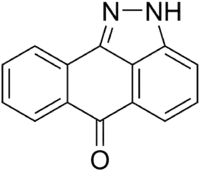
| Preferred IUPAC name
Dibenzo[cd,g]indazol-6(2H)-one | |
| Other names
Anthra[1,9-cd]pyrazol-6(2H)-one; Pyrazolanthrone; Pyrazoleanthrone; SP 600125; C.I. 70300; NSC 75890 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.004.506 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H8N2O | |
| Molar mass | 220.231 g·mol−1 |
| Appearance | yellow |
| Density | 1.463 g cm−3 |
| Melting point | 281 to 282 °C (538 to 540 °F; 554 to 555 K) |
| insoluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
1,9-Pyrazoloanthrone is a chemical compound that is a derivative of anthrone. It is used in biochemical studies as an inhibitor of c-Jun N-terminal kinases (JNKs).[1][2]
Derivatives of 1,9-pyrazoloanthrone have a variety of biological activities. For example, 5-(aminoalkyl)amino derivatives have been investigated as anticancer agents.[3]
Synthesis
1,9-Pyrazoloanthrone can be synthesized by the condensation of 2-chloroanthraquinone with anhydrous hydrazine in pyridine at 100 °C. Purification is achieved via conversion to the N-acetyl derivative which is crystallized from acetic acid, followed by hydrolysis of the acetyl group with ammonium hydroxide in methanol.
References
- ↑ Okuno S, Saito A, Hayashi T, Chan PH (2004). "The c-Jun N-terminal protein kinase signaling pathway mediates Bax activation and subsequent neuronal apoptosis through interaction with Bim after transient focal cerebral ischemia". J. Neurosci. 24 (36): 7879–87. doi:10.1523/JNEUROSCI.1745-04.2004. PMC 6729938. PMID 15356200.
- ↑ Bennett, B. L.; Sasaki, D. T.; Murray, B. W.; O'Leary, E. C.; Sakata, S. T.; Xu, W.; Leisten, J. C.; Motiwala, A.; Pierce, S.; Satoh, Y.; Bhagwat, S. S.; Manning, A. M.; Anderson, D. W. (2001). "SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase". Proceedings of the National Academy of Sciences. 98 (24): 13681–13686. Bibcode:2001PNAS...9813681B. doi:10.1073/pnas.251194298. PMC 61101. PMID 11717429.
- ↑ Showalter HD, Johnson JL, Werbel LM, Leopold WR, Jackson RC, Elslager EF (1984). "5-[(Aminoalkyl)amino]-substituted anthra[1,9-cd]pyrazol-6(2H)-ones as novel anticancer agents. Synthesis and biological evaluation". J. Med. Chem. 27 (3): 253–5. doi:10.1021/jm00369a002. PMID 6699870.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.