| Armadillo repeat domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| Symbol | Arm | ||||||||
| Pfam | PF00514 | ||||||||
| Pfam clan | CL0020 | ||||||||
| InterPro | IPR000225 | ||||||||
| SMART | SM00185 | ||||||||
| PROSITE | PS50176 | ||||||||
| SCOP2 | 3bct / SCOPe / SUPFAM | ||||||||
| CDD | cd00020 | ||||||||
| Membranome | 350 | ||||||||
| |||||||||
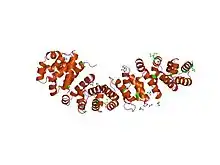
An armadillo repeat is a characteristic, repetitive amino acid sequence of about 42 residues in length that is found in many proteins. Proteins that contain armadillo repeats typically contain several tandemly repeated copies.[2][3] Each armadillo repeat is composed of a pair of alpha helices that form a hairpin structure. Multiple copies of the repeat form what is known as an alpha solenoid structure.
Examples of proteins that contain armadillo repeats include β-catenin, Sarm1 (SARM1),[4] α-importin,[5] plakoglobin,[6] adenomatous polyposis coli (APC),[7] and many others.
The term armadillo derives from the historical name of the β-catenin gene in the fruitfly Drosophila where the armadillo repeat was first discovered. Although β-catenin was previously believed to be a protein involved in linking cadherin cell adhesion proteins to the cytoskeleton, recent work indicates that β-catenin regulates the homodimerization of alpha-catenin, which in turn controls actin branching and bundling.[8] But, the armadillo repeat is found in a wide range of proteins with other functions. This type of protein domain is important in transducing WNT signals during embryonic development.
Structure
The 3-dimensional fold of an armadillo repeat was first observed in the crystal structure of β-catenin, where the 12 tandem repeats form a superhelix of alpha helices with three helices per unit.[1] The cylindrical structure features a positively charged groove, which presumably interacts with the acidic surfaces of the known interaction partners of β-catenin.[9]
References
- 1 2 Huber AH, Nelson WJ, Weis WI (September 1997). "Three-dimensional structure of the armadillo repeat region of β-catenin". Cell. 90 (5): 871–82. doi:10.1016/S0092-8674(00)80352-9. PMID 9298899. S2CID 18612343.
- ↑ Peifer M, Berg S, Reynolds AB (1994). "A repeating amino acid motif shared by proteins with diverse cellular roles". Cell. 76 (5): 789–91. doi:10.1016/0092-8674(94)90353-0. PMID 7907279. S2CID 26528190.
- ↑ Groves MR, Barford D (1999). "Topological characteristics of helical repeat proteins". Current Opinion in Structural Biology. 9 (3): 383–9. doi:10.1016/S0959-440X(99)80052-9. PMID 10361086.
- ↑ "Scopus preview - Scopus - Welcome to Scopus". www.scopus.com. Retrieved 2023-03-21.
- ↑ Herold A, Truant R, Wiegand H, Cullen BR (October 1998). "Determination of the functional domain organization of the importin alpha nuclear import factor". J. Cell Biol. 143 (2): 309–18. doi:10.1083/jcb.143.2.309. PMC 2132842. PMID 9786944.
- ↑ McCrea PD, Turck CW, Gumbiner B (November 1991). "A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin". Science. 254 (5036): 1359–61. Bibcode:1991Sci...254.1359M. doi:10.1126/science.1962194. PMID 1962194.
- ↑ Hirschl D, Bayer P, Müller O (March 1996). "Secondary structure of an armadillo single repeat from the APC protein". FEBS Lett. 383 (1–2): 31–6. doi:10.1016/0014-5793(96)00215-3. PMID 8612785. S2CID 36190869.
- ↑ Nusse, Roel, and Hans Clevers. “Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities.” Cell, vol. 169, no. 6, 1 June 2017, pp. 985–999., doi:10.1016/j.cell.2017.05.016.
- ↑ "Armadillo (IPR000225)". InterPro. EMBL-EBI.
External links
- Eukaryotic Linear Motif resource motif class TRG_NLS_Bipartite_1
- Eukaryotic Linear Motif resource motif class TRG_NLS_MonoCore_2
- Eukaryotic Linear Motif resource motif class TRG_NLS_MonoExtC_3
- Eukaryotic Linear Motif resource motif class TRG_NLS_MonoExtN_4
- Armadillo+Domain+Proteins at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Armadillo/plakoglobin ARM repeat in PROSITE