 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Augtyro |
| Other names | TPX-0005 |
| AHFS/Drugs.com | Augtyro |
| License data | |
| Routes of administration | By mouth |
| Drug class | Tyrosine kinase inhibitor |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
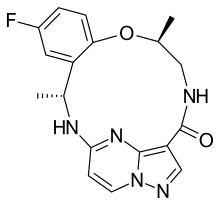
| Formula | C18H18FN5O2 |
| Molar mass | 355.373 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Repotrectinib, sold under the brand name Augtyro, is an anti-cancer medication used for the treatment of non-small cell lung cancer.[1][2] It is taken by mouth.[1] Repotrectinib is an inhibitor of proto-oncogene tyrosine-protein kinase ROS1 (ROS1) and of the tropomyosin receptor tyrosine kinases (TRKs) TRKA, TRKB, and TRKC.[1]
The most common adverse reactions include dizziness, dysgeusia, peripheral neuropathy, constipation, dyspnea, ataxia, fatigue, cognitive disorders, and muscular weakness.[2]
Repotrectinib was approved for medical use in the United States in November 2023.[2][3]
Medical uses
Repotrectinib is indicated for the treatment of adults with locally advanced or metastatic ROS1-positive non-small cell lung cancer.[1][2]
History
Approval by the US Food and Drug Administration (FDA) was based on TRIDENT-1, a global, multicenter, single-arm, open-label, multi-cohort clinical trial (NCT03093116) which included participants with ROS1-positive locally advanced or metastatic non-small cell lung cancer.[2] Efficacy was evaluated in 71 ROS1 tyrosine kinase inhibitor-naïve participants who received up to one prior line of platinum-based chemotherapy and/or immunotherapy and 56 participants who received one prior ROS1 tyrosine kinase inhibitor with no prior platinum-based chemotherapy or immunotherapy.[2]
The FDA granted the application for repotrectinib priority review, breakthrough therapy, and fast track designations.[2]
References
- 1 2 3 4 5 "Augtyro- repotrectinib capsule". DailyMed. 15 November 2023. Archived from the original on 12 December 2023. Retrieved 12 December 2023.
- 1 2 3 4 5 6 7 "FDA approves repotrectinib for ROS1-positive non-small cell lung cancer". U.S. Food and Drug Administration (FDA). 15 November 2023. Archived from the original on 16 November 2023. Retrieved 17 November 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "U.S. Food and Drug Administration Approves Augtyro (repotrectinib), a Next-Generation Tyrosine Kinase Inhibitor (TKI), for the Treatment of Locally Advanced or Metastatic ROS1-Positive Non-Small Cell Lung Cancer (NSCLC)" (Press release). Bristol Myers Squibb. 16 November 2023. Archived from the original on 16 November 2023. Retrieved 17 November 2023 – via Business Wire.
Further reading
- Drilon A, Ou SI, Cho BC, Kim DW, Lee J, Lin JJ, et al. (October 2018). "Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibits ROS1/TRK/ALK Solvent- Front Mutations". Cancer Discovery. 8 (10): 1227–1236. doi:10.1158/2159-8290.CD-18-0484. PMID 30093503.
External links
- "Repotrectinib (Code C133821)". NCI Thesaurus. 25 September 2023. Retrieved 17 November 2023.
- Clinical trial number NCT03093116 for "A Study of Repotrectinib (TPX-0005) in Patients With Advanced Solid Tumors Harboring ALK, ROS1, or NTRK1-3 Rearrangements (TRIDENT-1)" at ClinicalTrials.gov