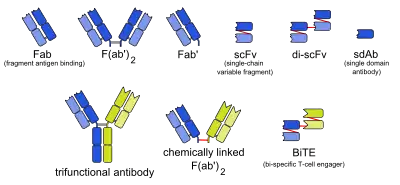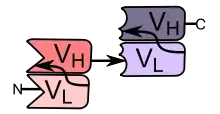
Bi-specific T-cell engagers (BiTEs) are a class of artificial bispecific monoclonal antibodies that are investigated for use as anti-cancer drugs. They direct a host's immune system, more specifically the T cells' cytotoxic activity, against cancer cells. BiTE is a registered trademark of Micromet AG (fully owned subsidiary of Amgen Inc).[1]
BiTEs are fusion proteins consisting of two single-chain variable fragments (scFvs) of different antibodies, or amino acid sequences from four different genes, on a single peptide chain of about 55 kilodaltons. One of the scFvs binds to T cells via the CD3 receptor, and the other to a tumor cell via a tumor specific molecule.[2][3]
Mechanism of action
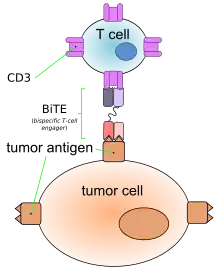
Like other bispecific antibodies, and unlike ordinary monoclonal antibodies, BiTEs form a link between T cells and tumor cells. This causes T cells to exert cytotoxic activity on tumor cells by producing proteins like perforin and granzymes, independently of the presence of MHC I or co-stimulatory molecules. These proteins enter tumor cells and initiate the cell's apoptosis.[2][4]
This action mimics physiological processes observed during T cell attacks against tumor cells.[4]
BiTEs in clinical assessment or with clinical approvals
Several BiTEs are currently in preclinical and clinical trials to assess their therapeutic efficacy and safety. [5]
Blinatumomab
Blinatumomab links T cells with CD19 receptors found on the surface of B cells. The Food and Drug Administration (US) and the European Medicines Agency approved this therapy for adults with Philadelphia chromosome-negative relapsed or refractory acute lymphoblastic leukemia.[6]
Glofitamab
It is a bispecific CD20-directed CD3 T-cell engager. It was approved for medical use in Canada in March 2023, in the United States in June 2023, and in the European Union in July 2023.
Mosunetuzumab
Bispecifically binds CD20 and CD3 to engage T-cells. Mosunetuzumab was approved for medical use in the European Union in June 2022.
Solitomab
Solitomab links T cells with the EpCAM antigen which is expressed by colon, gastric, prostate, ovarian, lung, and pancreatic cancers.[7][8]
Talquetamab
Tarlatamab
Tarlatamab has shown promise for previously treated small cell lung cancer.[9]
Tebentafusp
After clinical trials, in January 2022, the US FDA approved tebentafusp (a BiTE targeting the gp100 peptide) for HLA-A*02:01-positive adult patients with unresectable or metastatic uveal melanoma.[10]
Further research
Utilizing the same technology, melanoma (with MCSP specific BiTEs) and acute myeloid leukemia (with CD33 specific BiTEs) can be targeted.[11] As of 2008, research in this area is active.[11]
Another avenue for novel anti-cancer therapies is re-engineering some of the currently used conventional antibodies like trastuzumab (targeting HER2/neu), cetuximab and panitumumab (both targeting the EGF receptor), using the BiTE approach.[12]
As of 2009, BiTEs against CD66e and EphA2 are being developed as well.[13]
References
- ↑ "US Trademark registration no. 3,068,856, serial number 78/040,636". US Patent and Trademark Office.
- 1 2 Helwick, Caroline (1 June 2008). "Novel BiTE antibody mediates contact between T cells and cancer cells". Oncology NEWS International. 17 (6).
- ↑ Rüttinger, D.; Zugmaier, G.; Nagorsen, D.; Reinhardt, C.; Baeuerle, P. A. (2008). "BiTE-Antikörper: Durch Bispezifität T-Lymphozyten gegen Tumorzellen richten" [BiTE antibodies: Directing T lymphocytes against tumor cells by bispecifity]. Journal Onkologie (in German) (4).
- 1 2 "BiTE Antibody Platform". Micromet Inc.
- ↑ Voynov, V; Adam, PJ (2020). "Discovery Strategies to Maximize the Clinical Potential of T-Cell Engaging Antibodies for the Treatment of Solid Tumors". Antibodies. 9 (4): E65–E81. doi:10.3390/antib9040065. PMC 7709135. PMID 33217946. S2CID 227100306.
- ↑ Malard, Florent; Mohty, Mohamad (4 April 2020). "Acute lymphoblastic leukaemia". The Lancet. 395 (10230): 1146–1162. doi:10.1016/s0140-6736(19)33018-1. ISSN 0140-6736. PMID 32247396. S2CID 214779717.
- ↑ Amann, M.; d'Argouges, S.; Lorenczewski, G.; Brischwein, K.; Kischel, R.; Lutterbuese, R.; Mangold, S.; Rau, D.; Volkland, J.; Pflanz, S.; Raum, T.; Münz, M.; Kufer, P.; Schlereth, B.; Baeuerle, P. A.; Friedrich, M. (2009). "Antitumor Activity of an EpCAM/CD3-bispecific BiTE Antibody During Long-term Treatment of Mice in the Absence of T-cell Anergy and Sustained Cytokine Release". Journal of Immunotherapy. 32 (5): 452–464. doi:10.1097/CJI.0b013e3181a1c097. PMID 19609237. S2CID 25568468.
- ↑ Kebenko, Maxim; Goebeler, Marie-Elisabeth; Wolf, Martin; Hasenburg, Annette; Seggewiss-Bernhardt, Ruth; Ritter, Barbara; Rautenberg, Beate; Atanackovic, Djordje; Kratzer, Andrea; Rottman, James B.; Friedrich, Matthias (2018). "A multicenter phase 1 study of solitomab (MT110, AMG 110), a bispecific EpCAM/CD3 T-cell engager (BiTE®) antibody construct, in patients with refractory solid tumors". Oncoimmunology. 7 (8): e1450710. doi:10.1080/2162402X.2018.1450710. ISSN 2162-4011. PMC 6136859. PMID 30221040.
- ↑ Ahn, Myung-Ju; Cho, Byoung Chul; Felip, Enriqueta; Korantzis, Ippokratis; Ohashi, Kadoaki; Majem, Margarita; Juan-Vidal, Oscar; Handzhiev, Sabin; Izumi, Hiroki; Lee, Jong-Seok; Dziadziuszko, Rafal; Wolf, Jürgen; Blackhall, Fiona; Reck, Martin; Bustamante Alvarez, Jean (2023-11-30). "Tarlatamab for Patients with Previously Treated Small-Cell Lung Cancer". New England Journal of Medicine. 389 (22): 2063–2075. doi:10.1056/NEJMoa2307980. ISSN 0028-4793.
- ↑ Research, Center for Drug Evaluation and (January 26, 2022). "FDA approves tebentafusp-tebn for unresectable or metastatic uveal melanoma". FDA.
- 1 2 Kischel, R; et al. (2008). "Characterization in primates of MCSP- and CD33-specific human BiTE antibodies for treatment of Melanoma and AML" (PDF). Proc Am Assoc Cancer Res. 99. Abs 2404. Archived from the original (PDF) on 2011-07-19.
- ↑ Lutterbuese, R; et al. (2008). "Conversion of cetuximab, panitumumab, trastuzumab and omalizumab into T-cell-engaging BiTE antibodies creates novel drug candidates of high potency" (PDF). Proc Am Assoc Cancer Res. 99. Abs 2402. Archived from the original (PDF) on 2011-07-19.
- ↑ Baeuerle, PA; Reinhardt, C (2009). "Bispecific T-cell engaging antibodies for cancer therapy". Cancer Research. 69 (12): 4941–4. doi:10.1158/0008-5472.CAN-09-0547. PMID 19509221.
Further reading
- Kufer, P; Lutterbüse, R; Baeuerle, PA (2004). "A revival of bispecific antibodies". Trends in Biotechnology. 22 (5): 238–44. doi:10.1016/j.tibtech.2004.03.006. PMID 15109810.