| Boquila | |
|---|---|
.jpg.webp) | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Eudicots |
| Order: | Ranunculales |
| Family: | Lardizabalaceae |
| Genus: | Boquila Decne. |
| Species: | B. trifoliolata |
| Binomial name | |
| Boquila trifoliolata | |
| Synonyms[1] | |
| |
Boquila is a monotypic genus of flowering plants in the family Lardizabalaceae, endemic to temperate forests of central and southern Chile and Argentina. The sole species is Boquila trifoliolata, the chameleon vine. The sole member of this genus was first described in 1782 by Juan Ignacio Molina, and the genus itself was established in 1839 by Joseph Decaisne. B. trifoliata forms non-parasitic vines that wind around host plants, using them for structure and protection. B. trifoliata is monoecious, and its flowers are an off white color. It bears an edible fruit and has been historically used in rope and basket making.
B. trifoliata is the only known plant species to engage in mimetic polymorphism, or the ability to mimic multiple host species, often simultaneously. This is a form of Batesian mimicry, when a harmless species mimics a harmful one to ward off predators.Contact between the vines and host trees is not necessary for mimicking to commence, and the mechanism by which this occurs is still unknown. Hypotheses about the mechanism include microbial mediated horizontal gene transfer, volatile organic compound sensing, and the use of eye-like structures.
Taxonomy and etymology
Boquila is a monotypic genus of flowering plants (angiosperms) in the family Lardizabalaceae. The only known member of this genus is Boquila trifoliolata.[2][3] The species was first described as Dolichos funarius in 1782 by Juan Ignacio Molina, and in 1817, the holotype Lardizabala trifoliolata was named by Augustin Pyramus de Candolle.[4][5] In 1838, Stephan Endlicher, Eduard Friedrich Poeppig, and Gustav Kunze proposed the name Lardizabala discolor. In between 1837 to 1839, Joseph Decaisne identified Boquila trifoliolata and Boquila discolor, and established the Boquila genus in 1837.[6] The name Boquila discolor was later declared a orthographic variant. In 1936, Gualterio Looser attempted to reclassify the species to Lardizabala funaria based upon the observations of Carlo Giuseppe Bertero, but this classification is not considered valid.[7][8]
Due to its mimicry capabilities, Boquila trifoliolata is sometimes referred to as the Chameleon Vine.[9][10][11] Boquila trifoliolataand other Chilean climbing plants are collectively referred to as ‘voqui’ [‘bo.ki], a term that derives from the Mapuche word for liana.[12]
Description
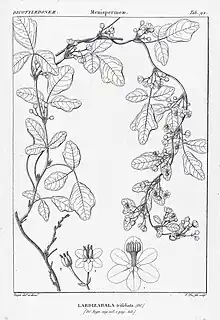
_2.jpg.webp)
Boquila trifoliolata, the sole member of the Boquila genus, is a woody vine with a highly variable appearance due to its crypsis abilities.[3] The vines are evergreen or partly deciduous, meaning they largely retain their leaves over winter.[12] The vines follow a twining pattern when climbing host plants, meaning the stems bend around host plants during their ascent.[13] The branches are thin, less than 1 cm (0.39 in) in diameter, and are covered in red-brown bark. The lenticels are elliptical in shape, and the wider branches are a speckled grey color.[12] When not mimicking a host plant, B. trifoliata employs smaller 'charlatan leaves' that are short, stubby, and have three lobes (trifoliate).[3] The petioles range from 2 cm (0.79 in) to 6 cm (2.4 in) in length and the petiolules range from 0.5 cm (0.20 in) to 1.5 cm (0.59 in) in length. Leaflets are oval or elliptical and range from 2 cm (0.79 in) to 6 cm (2.4 in) in height and 1 cm (0.39 in) to 3 cm (1.2 in) in width. The base of the leaves is rounded, the margins are irregular (most often trilobate), the tips are rounded and wide-angled, the top of the leaves are dark green and hairless, the undersides are glaucous (pale-grey to blue-green), and the veins have a pinnate pattern.[14][12]
Reproduction
In the wild, flowering occurs between September and December, while fruiting occurs between January and March. This pattern is opposite when the plant is raised in the Northern Hemisphere.[12] B. trifoliata is monoecious, meaning that both male and female plants are needed in reproduction.[12] The petals are small (1.5 cm (0.59 in) to 3 cm (1.2 in) in length) and have a green-white to yellow-white color. These flowers tend to be in 2- to 4-flower umbels with small hairs and lepidote bracts along the petals. Each flower has six sepals, and are biserate, petaloid, ovate, and the three inner sepals are larger than the outer ones. Staminate flowers (male flowers) have six stamens, petals in an opposite pattern, and anthers are oblate. Carpellate flowers (female flowers) have six conical staminodes, three carpels, an elongated stigma, and sutures running vertically up the petals.[14][12]
The fruits are small, ranging from 0.5 cm (0.20 in) to 1 cm (0.39 in) in diameter, and white. There are typically 1-4 seeds per berry, ranging from 2.5 mm (0.098 in) to 5 mm (0.20 in). The seeds are oval, brown, and contain large amounts of endosperm.[12] Seeds are largely dispersed via animal vectors and readily germinate when planted.[14][15]
Mimicry
Boquila trifoliolata is the only plant known to engage in mimetic polymorphism, meaning it can mimic the leaves of multiple host plants.[3][16] Other species of vines are capable of limited crypsis for one host species, but B. trifoliata is notable since it can mimic the leaves of multiple species, with one vine capable of simultaneously mimicking multiple hosts. Mimetic polymorphism is only observed elsewhere in some species of butterflies, but that is the result of genetic divergence, unlike B. trifoliata which engages in rapid changes in leaf morphology.[3]
Once the vines approach a host tree's branches, the leaves begin to change their size, shape, color, vein patterns, spines, and orientation to match the host plant; sometimes expanding to 10x their original size.[3] B. trifoliata has been observed mimicking over 20 different species of plants.[17] These include native species such as Luma apiculata, Cissus striata, and Rhaphithamnus spinosus but also non-native species such as Ranunculus repens.[18]
Unlike most other mimicking species, close proximity is enough to induce mimicry and contact isn't required.[3] In one controversial study, B. trifoliata has been noted to mimic the leaves of plastic plants.[19] If the vines approach another tree, the vine begins simultaneously mimicking that species as well.[3] Mimicry is largely confined to the leaves closest to the host, meaning that sections of the vine approximately 60 cm (24 in) away from the host retain the non-mimicking phenotype.[20] This is a form of Batesian mimicry, where the B. trifoliata is harmless but resembles a less palatable or harmful plant to ward off herbivory species and pests.[21][22][3]
Possible explanations
The exact mechanism by which mimicry occurs is not well understood but may involve chemical, odor, genetic, metagenomic, transcriptomic, proteomic, metabolomic, epigenetic, and/or microbial cues to identify and mimic the species it is attached to.[20][3]
Volatile organic compounds
Plant ecologist Ernesto Gianoli proposed that the host tree may be emitting volatile organic compounds (VOCs) into the environment that B. trifoliata can detect.[3] The use of VOC-mediated plant-to-plant communication is widely employed in non-specific biological processes, including up-regulation of defense-related genes, and could explain why no contact is necessary for mimicry. Criticisms of this hypothesis are that this would mark the first time that VOCs were used to change plant morphology, and that B. trifoliata's mimicry has a level of specificity that is not normally seen with VOC-mediated responses.[20]
Horizontal gene transfer
Another hypothesis proposed by Gianoli is that B. trifoliata's mimicry is mediated by endophytic microbes that conduct horizontal gene transfer (HGT) between B. trifoliata and the host plant. This would influence the genes, transposons, and/or epigenetics of the plant's leaves, identifying the host and changing the leaf's morphology without necessitating physical contact.[18][19] In a 2021 study, Gianoli found that the microbiomes of B. trifoliata and its host plant show significant overlap following the initiation of mimicry. Gianoli has argued this could represent a mechanism behind B. trifoliata's mimicry but still acknowledged that there are limitations to this hypothesis. While HGT commonly occurs between different species, it takes many years and manifests in discrete events. Additionally, HGT between plants is most commonly observed in cases of parasitism, which B. trifoliata does not engage in.[18]
Ocelli
In a 2021 study published in the journal Plant Signaling & Behavior, Felipe Yamashita and Jacob White claimed that B. trifoliata may employ a primitive form of vision to identify and mimic their hosts. This hypothesis is based upon 1905 and 1907 claims by Gottlieb Haberlandt and Francis Darwin, respectively, that some plants use 'ocelli' or lens-like cells to focus light onto other light sensitive cells. In this study, B. trifoliata was observed mimicking the leaf shapes of plastic plants, and researchers refined Haberlandt and Darwin's ocelli hypothesis, claiming that B. trifoliata may be using convex shaped lenses in epidermal tissue that can detect light and "see" the shapes of nearby leaves.[23] They further proposed that, B. trifoliata processes that information through an unknown means, possibly through neuron-like structures in order to initiate mimicry.[17][19] The study also found that non-mimetic leaves have more free-end veinlets and identified the hormone auxin as a possible mediator in changes to leaf morphology.[23]
This paper received substantial media coverage, was praised by F1000's Faculty Opinions, and went viral on the social media platform TikTok following its release. Plant biologist and Plant Signaling and Behavior's editor-in-chief, František Baluška praised this hypothesis, and claimed that root skototropism and photoreceptive cells in algae were analogous mechanisms for "plant sight". However, the paper's conclusions have largely been met with skepticism by scientists. Criticisms of the paper include poor methodology, White's lack of a scientific background, and possible conflicts of interest between Baluška and Yamashita.[17][19]
Distribution and ecology
_1.jpg.webp)
The Boquila genus is endemic to the temperate rainforests, nothofagus forests, and evergreen forests of southern Argentina and Chile, ranging from Cauquenes to Chiloe.[12][3] B. trifoliata is most commonly found between 100 metres (330 ft) to 600 metres (2,000 ft) in elevation.[12] Unlike many other species of vines, B. trifoliata is not parasitic. Instead, it only attaches to trees for protection and structure, sometimes forming thickets over 6 metres (20 ft) in height. B. trifoliata can survive temperatures as low as −8 °C (18 °F) and prefers soil rich in humus. The species is resistant to wilting, but generally prefers to grow in shaded environments.[3][14][12]
Human uses
The stems are used locally in basketry and in rope making. The leaf juice was historically used by local tribes to treat sore eyes and was once believed to be an aphrodisiac.The plant is also used ornamentally and the berries are edible.[12] Stems are often cut in the summer and rooted in cold frames as a means of propagation.[14]
See also
- Lardizabala, a related species also grown for its fruit
References
- ↑ "The Plant List: A Working List of All Plant Species". Archived from the original on May 24, 2019. Retrieved June 19, 2014.
- ↑ taxonomy. "Taxonomy browser (Boquila trifoliolata)". www.ncbi.nlm.nih.gov. Archived from the original on 2023-12-09. Retrieved 2023-12-09.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Gianoli, Ernesto; Carrasco-Urra, Fernando (2014). "Leaf Mimicry in a Climbing Plant Protects against Herbivory". Current Biology. 24 (9): P984-987. doi:10.1016/j.cub.2014.03.010. PMID 24768053.
- ↑ Molina, Giovanni Ignazio (1782). "Saggio sulla storia naturale del Chili". Biodiversity Heritage Library. Retrieved 2024-01-04.
- ↑ Candolle, Augustin Pyramus de; Candolle, Augustin Pyramus de (1818). Regni vegetabilis systema naturale, sive Ordines, genera et species plantarum secundum methodi naturalis normas digestarum et descriptarum. Vol. v.1 (1818). Parisiis [etc.]: sumptibus sociorum Treuttel et Würtz.
- ↑ Compt. Rend. Hebd. Séances Acad. Sci. 3: 394 (1837)
- ↑ Lista Pls. Obs. Chile en 1828 por Bertero 36. 1936.
- ↑ Zuloaga, F. O., O. Morrone, M. J. Belgrano, C. F. S. Marticorena & E. Marchesi. (eds.) 2008. Catálogo de las plantas vasculares del Cono Sur. Monogr. Syst. Bot. Missouri Bot. Gard. 107(1–3)
- ↑ Yang, Ina (2014-05-07). "'Chameleon' Vine Looks Like Whatever Tree It Climbs". Popular Science. Archived from the original on 2023-12-09. Retrieved 2023-12-09.
- ↑ "ScienceShot: 'Chameleon' Vine Discovered in Chile". Science | AAAS. 2014-04-24. Archived from the original on 2021-10-31. Retrieved 2018-06-02.
- ↑ Puiu, Tibi (2014-04-25). "The Chameleon vine: the only plant that morphs host plants near it". ZME Science. Archived from the original on 2023-12-09. Retrieved 2023-12-09.
- 1 2 3 4 5 6 7 8 9 10 11 12 Christenhusz, Maarten J. M. (2012). "738. Boquila Trifoliolata". Curtis's Botanical Magazine. 29 (3): 277–283. doi:10.1111/j.1467-8748.2012.01791.x. ISSN 1355-4905. Archived from the original on 2023-12-13. Retrieved 2023-12-13.
- ↑ Valladares, Fernando, Ernesto Gianoli, and Alfredo Saldana. "Climbing plants in a temperate rainforest understorey: searching for high light or coping with deep shade?." Annals of Botany 108.2 (2011): 231-239.
- 1 2 3 4 5 Christenhusz, Maarten J. M. (2012). "An Overview of Lardizabalaceae". Curtis's Botanical Magazine. 29 (3): 235–276. doi:10.1111/j.1467-8748.2012.01790.x. ISSN 1355-4905. Archived from the original on 2022-11-22. Retrieved 2023-12-14.
- ↑ Vazquez, Miriam Soledad; Rodriguez‐Cabal, Mariano A.; Amico, Guillermo C. (March 2022). "The forest gardener: A marsupial with a key seed‐dispersing role in the Patagonian temperate forest". Ecological Research. 37 (2): 270–283. doi:10.1111/1440-1703.12289. ISSN 0912-3814. S2CID 245597896. Archived from the original on 2023-12-14. Retrieved 2023-12-14.
- ↑ Yirka, Bob; Phys.org. "Researchers discover vine that is able to mimic multiple hosts". phys.org. Retrieved 2024-01-10.
- 1 2 3 Wilcox, Christie. "Can Plants See? In the Wake of a Controversial Study, the Answer's Still Unclear". The Scientist Magazine. Archived from the original on 2023-12-09. Retrieved 2023-12-09.
- 1 2 3 Gianoli, Ernesto; González-Teuber, Marcia; Vilo, Claudia; Guevara-Araya, María J.; Escobedo, Víctor M. (2021-11-22). "Endophytic bacterial communities are associated with leaf mimicry in the vine Boquila trifoliolata". Scientific Reports. 11 (1): 22673. doi:10.1038/s41598-021-02229-8. ISSN 2045-2322. PMC 8608808. PMID 34811460.
- 1 2 3 4 Jones, Benji (2022-11-30). "The mystery of the mimic plant". Vox. Archived from the original on 2023-09-21. Retrieved 2023-12-09.
- 1 2 3 Gianoli, Ernesto; González-Teuber, Marcia; Vilo, Claudia; Guevara-Araya, María J.; Escobedo, Víctor M. (2021-11-22). "Endophytic bacterial communities are associated with leaf mimicry in the vine Boquila trifoliolata". Scientific Reports. 11 (1): 22673. doi:10.1038/s41598-021-02229-8. ISSN 2045-2322. PMC 8608808. PMID 34811460.
- ↑ Taylor, Christopher H. (2023-04-01). "Body size in Batesian mimicry". Evolutionary Ecology. 37 (2): 233–243. doi:10.1007/s10682-022-10204-6. ISSN 1573-8477.
- ↑ Krulwich, Robert (2016-02-19). "The Sneaky Life of the World's Most Mysterious Plant". National Geographic. Archived from the original on 2023-12-09. Retrieved 2023-12-09.
- 1 2 White, Jacob; Yamashita, Felipe (2022-12-31). "Boquila trifoliolata mimics leaves of an artificial plastic host plant". Plant Signaling & Behavior. 17 (1). doi:10.1080/15592324.2021.1977530. ISSN 1559-2324. PMC 8903786. PMID 34545774.