| COL10A1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | COL10A1, collagen type X alpha 1 chain | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 120110 MGI: 88445 HomoloGene: 55466 GeneCards: COL10A1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Collagen alpha-1(X) chain is a protein that in humans is a member of the collagen family encoded by the COL10A1 gene.[5][6]
This gene encodes the alpha chain of type X collagen, a short chain collagen expressed by hypertrophic chondrocytes during endochondral ossification. Unlike type VIII collagen, the other short chain collagen, type X collagen is a homotrimer. Type X collagen has a short triple helical collagen domain flanked by the N-terminal NC2 and the C-terminal NC1 domains. The C-terminal NC1 domain has complement C1q-like structure. Collagen X forms hexamer complexes through the association of NC1 regions.[7] Mutations in this gene are associated with Schmid type metaphyseal chondrodysplasia (SMCD) and Japanese type spondylometaphyseal dysplasia (SMD).[6]
DDR2 is a collagen receptor for it.[8]
Recent studies into the early detection of colon cancer have identified COL10A1 protein levels in serum as a potential diagnostic biomarker candidate to detect both adenoma lesions and tumor.[9]
Collagen alpha-1(X) undergoes degradation in the active growth plate releasing an intact NC1 region with a small amount of collagenous region attached. This degradation byproduct has been deemed CXM and has potential to be a useful biomarker to assess real time growth velocity in children and fracture healing in adults.[10]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000123500 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000039462 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
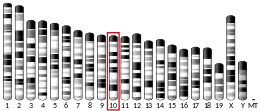
- ↑ Apte S, Mattei MG, Olsen BR (Jul 1991). "Cloning of human alpha 1(X) collagen DNA and localization of the COL10A1 gene to the q21-q22 region of human chromosome 6". FEBS Lett. 282 (2): 393–6. doi:10.1016/0014-5793(91)80521-4. PMID 2037056. S2CID 6753444.
- 1 2 "Entrez Gene: COL10A1 collagen, type X, alpha 1(Schmid metaphyseal chondrodysplasia)".
- ↑ Kwan et al. 2005
- ↑ Leitinger B, Kwan AP (August 2006). "The discoidin domain receptor DDR2 is a receptor for type X collagen". Matrix Biol. 25 (6): 355–64. doi:10.1016/j.matbio.2006.05.006. PMID 16806867.
- ↑ Solé X, Crous-Bou M, Cordero D, Olivares D, Guinó E, Sanz-Pamplona R, Rodriguez-Moranta F, Sanjuan X, de Oca J, Salazar R, Moreno V (September 2014). "Discovery and validation of new potential biomarkers for early detection of colon cancer". PLOS ONE. 9 (9): e106748. Bibcode:2014PLoSO...9j6748S. doi:10.1371/journal.pone.0106748. PMC 4162553. PMID 25215506.
- ↑ Coghlan, Ryan F.; Oberdorf, Jon A.; Sienko, Susan; Aiona, Michael D.; Boston, Bruce A.; Connelly, Kara J.; Bahney, Chelsea; Larouche, Jeremie; Almubarak, Sarah M.; Coleman, Daniel T.; Girkontaite, Irute; von Der Mark, Klaus; Lunstrum, Gregory P.; Horton, William A. (2017). "A degradation fragment of type X collagen is a real-time marker for bone growth velocity". Science Translational Medicine. 9 (419): eaan4669. doi:10.1126/scitranslmed.aan4669. PMC 6516194. PMID 29212713.
Further reading
- Kuivaniemi H, Tromp G, Prockop DJ (1997). "Mutations in fibrillar collagens (types I, II, III, and XI), fibril-associated collagen (type IX), and network-forming collagen (type X) cause a spectrum of diseases of bone, cartilage, and blood vessels". Hum. Mutat. 9 (4): 300–15. doi:10.1002/(SICI)1098-1004(1997)9:4<300::AID-HUMU2>3.0.CO;2-9. PMID 9101290. S2CID 6890740.
- Kirsch T, Pfäffle M (1992). "Selective binding of anchorin CII (annexin V) to type II and X collagen and to chondrocalcin (C-propeptide of type II collagen). Implications for anchoring function between matrix vesicles and matrix proteins". FEBS Lett. 310 (2): 143–7. doi:10.1016/0014-5793(92)81316-E. PMID 1397263. S2CID 9498732.
- Reichenberger E, Beier F, LuValle P, et al. (1992). "Genomic organization and full-length cDNA sequence of human collagen X." FEBS Lett. 311 (3): 305–10. doi:10.1016/0014-5793(92)81126-7. PMID 1397333. S2CID 12022346.
- Apte SS, Seldin MF, Hayashi M, Olsen BR (1992). "Cloning of the human and mouse type X collagen genes and mapping of the mouse type X collagen gene to chromosome 10". Eur. J. Biochem. 206 (1): 217–24. doi:10.1111/j.1432-1033.1992.tb16919.x. PMID 1587271.
- Reichenberger E, Aigner T, von der Mark K, et al. (1992). "In situ hybridization studies on the expression of type X collagen in fetal human cartilage". Dev. Biol. 148 (2): 562–72. doi:10.1016/0012-1606(91)90274-7. PMID 1743401.
- Thomas JT, Cresswell CJ, Rash B, et al. (1992). "The human collagen X gene. Complete primary translated sequence and chromosomal localization". Biochem. J. 280 (3): 617–23. doi:10.1042/bj2800617. PMC 1130499. PMID 1764025.
- Bonaventure J, Chaminade F, Maroteaux P (1995). "Mutations in three subdomains of the carboxy-terminal region of collagen type X account for most of the Schmid metaphyseal dysplasias". Hum. Genet. 96 (1): 58–64. doi:10.1007/BF00214187. PMID 7607655. S2CID 20888881.
- McIntosh I, Abbott MH, Francomano CA (1995). "Concentration of mutations causing Schmid metaphyseal chondrodysplasia in the C-terminal noncollagenous domain of type X collagen". Hum. Mutat. 5 (2): 121–5. doi:10.1002/humu.1380050204. PMID 7749409. S2CID 26291298.
- Chan D, Cole WG, Rogers JG, Bateman JF (1995). "Type X collagen multimer assembly in vitro is prevented by a Gly618 to Val mutation in the alpha 1(X) NC1 domain resulting in Schmid metaphyseal chondrodysplasia". J. Biol. Chem. 270 (9): 4558–62. doi:10.1074/jbc.270.9.4558. PMID 7876225.
- McIntosh I, Abbott MH, Warman ML, et al. (1994). "Additional mutations of type X collagen confirm COL10A1 as the Schmid metaphyseal chondrodysplasia locus". Hum. Mol. Genet. 3 (2): 303–7. doi:10.1093/hmg/3.2.303. PMID 8004099.
- Dharmavaram RM, Elberson MA, Peng M, et al. (1994). "Identification of a mutation in type X collagen in a family with Schmid metaphyseal chondrodysplasia". Hum. Mol. Genet. 3 (3): 507–9. doi:10.1093/hmg/3.3.507. PMID 8012364.
- Warman ML, Abbott M, Apte SS, et al. (1993). "A type X collagen mutation causes Schmid metaphyseal chondrodysplasia". Nat. Genet. 5 (1): 79–82. doi:10.1038/ng0993-79. PMID 8220429. S2CID 196834.
- Wallis GA, Rash B, Sweetman WA, et al. (1994). "Amino acid substitutions of conserved residues in the carboxyl-terminal domain of the alpha 1(X) chain of type X collagen occur in two unrelated families with metaphyseal chondrodysplasia type Schmid". Am. J. Hum. Genet. 54 (2): 169–78. PMC 1918153. PMID 8304336.
- Pokharel RK, Alimsardjono H, Uno K, et al. (1996). "A novel mutation substituting tryptophan with arginine in the carboxyl-terminal, non-collagenous domain of collagen X in a case of Schmid metaphyseal chondrodysplasia". Biochem. Biophys. Res. Commun. 217 (3): 1157–62. doi:10.1006/bbrc.1995.2890. hdl:20.500.14094/D1001967. PMID 8554571.
- Wallis GA, Rash B, Sykes B, et al. (1996). "Mutations within the gene encoding the alpha 1 (X) chain of type X collagen (COL10A1) cause metaphyseal chondrodysplasia type Schmid but not several other forms of metaphyseal chondrodysplasia". J. Med. Genet. 33 (6): 450–7. doi:10.1136/jmg.33.6.450. PMC 1050629. PMID 8782043.
- Stratakis CA, Orban Z, Burns AL, et al. (1997). "Dideoxyfingerprinting (ddF) analysis of the type X collagen gene (COL10A1) and identification of a novel mutation (S671P) in a kindred with Schmid metaphyseal chondrodysplasia". Biochem. Mol. Med. 59 (2): 112–7. doi:10.1006/bmme.1996.0075. PMID 8986632.
- Beier F, Eerola I, Vuorio E, et al. (1997). "Variability in the upstream promoter and intron sequences of the human, mouse and chick type X collagen genes". Matrix Biol. 15 (6): 415–22. doi:10.1016/S0945-053X(96)90160-2. PMID 9049979.
- Ikegawa S, Nakamura K, Nagano A, et al. (1997). "Mutations in the N-terminal globular domain of the type X collagen gene (COL10A1) in patients with Schmid metaphyseal chondrodysplasia". Hum. Mutat. 9 (2): 131–5. doi:10.1002/(SICI)1098-1004(1997)9:2<131::AID-HUMU5>3.0.CO;2-C. PMID 9067753. S2CID 22871274.