| CA3 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | CA3, CAIII, Car3, carbonic anhydrase 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 114750 MGI: 88270 HomoloGene: 31298 GeneCards: CA3 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Carbonic anhydrase 3 is an enzyme that in humans is encoded by the CA3 gene.[5]
Carbonic anhydrase III (CAIII) is a member of a multigene family (at least six separate genes are known) that encode carbonic anhydrase isozymes. These carbonic anhydrases are a class of metalloenzymes that catalyze the reversible hydration of carbon dioxide and are differentially expressed in a number of cell types. The expression of the CA3 gene is strictly tissue-specific and present at high levels in skeletal muscle and much lower levels in cardiac and smooth muscle. CA3 is insufficient in muscles of Myasthenia Gravis patients.[6] A proportion of carriers of Duchenne muscle dystrophy have a higher CA3 level than normal. Autoantibodies to CA3 have been found to be significantly higher in patients with rheumatoid arthritis, systemic lupus erythematosus, and type 1 diabetes.[7] The gene spans 10.3 kb and contains seven exons and six introns.[8]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000164879 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000027559 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ Shima K, Tashiro K, Hibi N, Tsukada Y, Hirai H (Jun 1983). "Carbonic anhydrase-III immunohistochemical localization in human skeletal muscle". Acta Neuropathologica. 59 (3): 237–9. doi:10.1007/BF00703210. PMID 6221502. S2CID 523577.
- ↑ Du AL, Ren HM, Lu CZ, Tu JL, Xu CF, Sun YA (Mar 2009). "Carbonic anhydrase III is insufficient in muscles of myasthenia gravis patients". Autoimmunity. 42 (3): 209–15. doi:10.1080/08916930802668610. PMID 19301202. S2CID 3135174.
- ↑ Liu C, Wei Y, Wang J, Pi L, Huang J, Wang P (Sep 2012). "Carbonic anhydrases III and IV autoantibodies in rheumatoid arthritis, systemic lupus erythematosus, diabetes, hypertensive renal disease, and heart failure". Clinical and Developmental Immunology. 2012: 354594. doi:10.1155/2012/354594. PMC 3461255. PMID 23049597.
- ↑ "Entrez Gene: CA3 carbonic anhydrase III, muscle specific".
Further reading
- Sly WS, Hu PY (1995). "Human carbonic anhydrases and carbonic anhydrase deficiencies". Annu. Rev. Biochem. 64: 375–401. doi:10.1146/annurev.bi.64.070195.002111. PMID 7574487.
- Carter N, Jeffery S, Shiels A, et al. (1980). "Characterization of human carbonic anhydrase III from skeletal muscle". Biochemical Genetics. 17 (9–10): 837–54. doi:10.1007/BF00504307. PMID 120192. S2CID 8616344.
- Oikarinen A, Vuori J, Autio P, et al. (1993). "Comparison of muscle-derived serum carbonic anhydrase III and myoglobin in dermatological patients: effects of isotretinoin treatment". Acta Dermato-Venereologica. 72 (5): 352–4. PMID 1361281.
- Oguni M, Setogawa T, Tanaka O, et al. (1992). "Immunohistochemical study of carbonic anhydrase III in the extraocular muscles of human embryos". Acta Anatomica. 144 (4): 316–9. doi:10.1159/000147322. PMID 1414196.
- Vuori J, Rasi S, Takala T, Väänänen K (1992). "Dual-label time-resolved fluoroimmunoassay for simultaneous detection of myoglobin and carbonic anhydrase III in serum". Clinical Chemistry. 37 (12): 2087–92. doi:10.1093/clinchem/37.12.2087. PMID 1764784.
- Shamsutdinov NSh, Islamov RI, Valuillin VV (1991). "[Carbonic anhydrase III--a marker of the myoepithelial cells of human salivary glands]". Biulleten' eksperimental'noĭ biologii i meditsiny. 111 (3): 320–1. PMID 1905166.
- Nork TM, Wallow IH, Sramek SJ, et al. (1990). "Immunocytochemical study of an eye with proliferative vitreoretinopathy and retinal tacks". Retina. 10 (1): 78–85. doi:10.1097/00006982-199001010-00015. PMID 1971453. S2CID 7418386.
- Lloyd J, Brownson C, Tweedie S, et al. (1987). "Human muscle carbonic anhydrase: gene structure and DNA methylation patterns in fetal and adult tissues". Genes & Development. 1 (6): 594–602. doi:10.1101/gad.1.6.594. PMID 2824285.
- Lloyd JC, Isenberg H, Hopkinson DA, Edwards YH (1986). "Isolation of a cDNA clone for the human muscle specific carbonic anhydrase, CAIII". Annals of Human Genetics. 49 (Pt 3): 241–51. doi:10.1111/j.1469-1809.1985.tb01698.x. PMID 3000276. S2CID 30747496.
- Lloyd J, McMillan S, Hopkinson D, Edwards YH (1986). "Nucleotide sequence and derived amino acid sequence of a cDNA encoding human muscle carbonic anhydrase". Gene. 41 (2–3): 233–9. doi:10.1016/0378-1119(86)90103-4. PMID 3086182.
- Wade R, Gunning P, Eddy R, et al. (1987). "Nucleotide sequence, tissue-specific expression, and chromosome location of human carbonic anhydrase III: the human CAIII gene is located on the same chromosome as the closely linked CAI and CAII genes". Proceedings of the National Academy of Sciences. 83 (24): 9571–5. doi:10.1073/pnas.83.24.9571. PMC 387182. PMID 3099285.
- Väänänen HK, Autio-Harmainen H (1987). "Carbonic anhydrase III: a new histochemical marker for myoepithelial cells". Journal of Histochemistry & Cytochemistry. 35 (6): 683–6. doi:10.1177/35.6.3106467. PMID 3106467.
- Nakagawa Y, Perentes E, Rubinstein LJ (1987). "Non-specificity of anti-carbonic anhydrase C antibody as a marker in human neurooncology". Journal of Neuropathology and Experimental Neurology. 46 (4): 451–60. doi:10.1097/00005072-198707000-00004. PMID 3110380. S2CID 101554.
- Edwards YH, Lloyd J, Parkar M, Povey S (1988). "The gene for human muscle specific carbonic anhydrase (CAIII) is assigned to chromosome 8". Annals of Human Genetics. 50 (Pt 1): 41–7. doi:10.1111/j.1469-1809.1986.tb01937.x. PMID 3122635. S2CID 22462255.
- Väänänen HK, Paloniemi M, Vuori J (1985). "Purification and localization of human carbonic anhydrase. III. Typing of skeletal muscle fibers in paraffin embedded sections". Histochemistry. 83 (3): 231–5. doi:10.1007/bf00953989. PMID 3930440. S2CID 20902453.
- Carter ND, Heath R, Jeffery S, Rodeck C (1982). "Fetal plasma carbonic anhydrase III in Duchenne dystrophy". Lancet. 1 (8262): 39–40. doi:10.1016/S0140-6736(82)92574-0. PMID 6119426. S2CID 32433390.
- Heath R, Schwartz MS, Brown IR, Carter ND (1983). "Carbonic anhydrase III in neuromuscular disorders". Journal of the Neurological Sciences. 59 (3): 383–8. doi:10.1016/0022-510X(83)90023-0. PMID 6410007. S2CID 8548497.
- Wåhlstrand T, Wistrand PJ (1980). "Carbonic anhydrase C in the human renal medulla". Upsala Journal of Medical Sciences. 85 (1): 7–17. doi:10.3109/03009738009179167. PMID 6770531.
- Jeffery S, Edwards Y, Carter N (1981). "Distribution of CAIII in fetal and adult human tissue". Biochemical Genetics. 18 (9–10): 843–9. doi:10.1007/BF00500117. PMID 6784712. S2CID 13229349.
External links
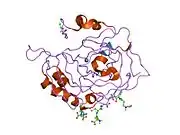
- Overview of all the structural information available in the PDB for UniProt: P07451 (Carbonic anhydrase 3) at the PDBe-KB.