 | |
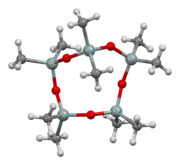 Ball-and-stick model of the molecule as found in the crystal structure determined by single-crystal X-ray diffraction[1] | |
 Sample of decamethylcyclopentasiloxane | |
| Names | |
|---|---|
| Preferred IUPAC name
Decamethyl-1,3,5,7,9,2,4,6,8,10-pentaoxapentasilecane | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| 1800166 | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.969 |
| EC Number |
|
| MeSH | Decamethylcyclopentasiloxane |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| [(CH3)2SiO]5 | |
| Molar mass | 370.770 g·mol−1 |
| Appearance | Colourless liquid |
| Density | 0.958 g/cm3 |
| Melting point | −47 °C; −53 °F; 226 K |
| Boiling point | 210 °C (410 °F; 483 K) |
| 17.03±0.72 ppb (23 °C) [2] | |
| log P | 8.07[3] |
| Vapor pressure | 20.4±1.1 Pa (25 °C) [4] |
| Viscosity | 3.74 cP |
| Hazards | |
| GHS labelling: | |
  | |
| Warning | |
| H226, H361, H412, H413 | |
| P201, P202, P210, P233, P240, P241, P242, P243, P273, P280, P281, P303+P361+P353, P308+P313, P370+P378, P403+P235, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 73 °C (163 °F; 346 K) |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Related Organosilicon compounds |
Octamethylcyclotetrasiloxane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
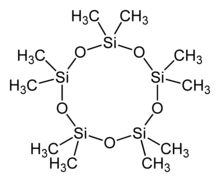
Decamethylcyclopentasiloxane, also known as D5 and D5, is an organosilicon compound with the formula [(CH3)2SiO]5. It is a colorless and odorless liquid that is slightly volatile.[5]
Use
The compound is classified as a cyclomethicone. Such fluids are commonly used in cosmetics, such as deodorants, sunblocks, hair sprays and skin care products. It is becoming more common in hair conditioners, as it makes the hair easier to brush without breakage. It is also used as part of silicone-based personal lubricants. D5 is considered an emollient. In Canada, among the volume used in consumer products approximately 70% were for antiperspirants and 20% for hair care products.[6] 10,000–100,000 tonnes per year of D5 is manufactured and/or imported in the European Economic Area.[7] Atmospheric emissions of D5 in the Northern Hemisphere were estimated to 30,000 tonnes per year.[8]
Decamethylcyclopentasiloxane has also been tried as a dry-cleaning solvent in the early 2000s. It was marketed as a more environmentally friendly solvent than tetrachloroethylene (the most common dry-cleaning solvent worldwide) despite being controlled in the EU for to its persistent, bioaccumulative and toxic characteristics.[9]
Production and polymerization
Commercially D5 is produced from dimethyldichlorosilane. Hydrolysis of the dichloride produces a mixture of cyclic dimethylsiloxanes and polydimethylsiloxane. From this mixture, the cyclic siloxanes including D5 can be removed by distillation. In the presence of a strong base such as KOH, the polymer/ring mixture is equilibrated, allowing complete conversion to the more volatile cyclic siloxanes:[10]
- [(CH3)2SiO]5n → n [(CH3)2SiO]5
where n is a positive integer. D4 and D5 are also precursors to the polymer. The catalyst is again KOH.
Safety and environmental considerations
The LD50 for D5 in rats is >50 g/kg.[10]
The environmental impacts of D5 and D4 have attracted attention because these compounds are pervasive. Cyclic siloxanes have been detected in some species of aquatic life.[11] A scientific review in Canada has determined that “Siloxane D5 does not pose a danger to the environment”[12] and a scientific assessment of D5 by the Australian government stated, "the direct risks to aquatic life from exposure to these chemicals at expected surface water concentrations are not likely to be significant."[13] However, in the European Union, D5 was characterized as a substance of very high concern (SVHC) due to its PBT and vPvB properties and was thus included in the candidate list for authorisation.[14] Since 31 January 2020, D5 cannot be placed on the market in the European Union in wash-off cosmetic products in a concentration equal to or greater than 0.1 % by weight.[15]
See also
References
- ↑ S. Parsons, D. Rankin, P. A. Wood (2004). "CSD Communication YAGTOQ: decamethylpentasilsesquioxane". Cambridge Structural Database: Access Structures. Cambridge Crystallographic Data Centre. doi:10.5517/cc89wzd. Retrieved 2021-08-18.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Sudarsanan Varaprath; Cecil L. Frye; Jerry Hamelink (1996). "Aqueous solubility of permethylsiloxanes (silicones)". Environmental Toxicology and Chemistry. 15 (8): 1263–1265. doi:10.1002/etc.5620150803.
- ↑ Shihe Xu, Bruce Kropscott (2012). "Method for Simultaneous Determination of Partition Coefficients for Cyclic Volatile Methylsiloxanes and Dimethylsilanediol". Analytical Chemistry. 84 (4): 1948–1955. doi:10.1021/ac202953t. PMID 22304371.
- ↑ Ying Duan Lei; Frank Wania; Dan Mathers (2010). "Temperature-Dependent Vapor Pressure of Selected Cyclic and Linear Polydimethylsiloxane Oligomers". Journal of Chemical & Engineering Data. 55 (12): 5868–5873. doi:10.1021/je100835n.
- ↑ Record of Decamethylcyclopentasiloxan in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 25. September 2015.
- ↑ Donald Mackay, Christina E. Cowan-Ellsberry, David E. Powell, Kent B. Woodburn, Shihe Xu, Gary E. Kozerski, Jaeshin Kim (2015). "Decamethylcyclopentasiloxane (D5) environmental sources, fate, transport, and routes of exposure". Environmental Toxicology and Chemistry. 34 (12): 2689–2702. doi:10.1002/etc.2941. PMID 26213270.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ "InfoCard – Decamethylcyclopentasiloxane". ECHA. Retrieved 2018-07-18.
- ↑ Michael S. McLachlan, Amelie Kierkegaard, Kaj M. Hansen, Roger van Egmond, Jesper H. Christensen, Carsten A. Skjøth (2010). "Concentrations and Fate of Decamethylcyclopentasiloxane (D5) in the Atmosphere". Environmental Science & Technology. 44 (14): 5365–5370. Bibcode:2010EnST...44.5365M. doi:10.1021/es100411w. PMID 20568739.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Commission Regulation (EU) 2018/35 of 10 January 2018 amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards octamethylcyclotetrasiloxane (‘D4’) and decamethylcyclopentasiloxane (‘D5’) (Text with EEA relevance. ), 2018-01-10, retrieved 2023-08-10
- 1 2 Moretto, Hans-Heinrich; Schulze, Manfred; Wagner, Gebhard (2005). "Silicones". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a24_057. ISBN 3527306730.
- ↑ Wang, De-Gao; Norwood, Warren; Alaee, Mehran; Byer, Jonathan D.; Brimble, Samantha: Review of recent advances in research on the toxicity, detection, occurrence and fate of cyclic volatile methyl siloxanes in the environment, Chemosphere 2013, volume 93, pages 711–725. doi:10.1016/j.chemosphere.2012.10.041
- ↑ Report of the Board of Review for Decamethylcyclopentasiloxane (Siloxane D5) established under Section 333(1) of the Canadian Environmental Protection Act of 1999, October 20, 2011
- ↑ "Cyclic volatile methyl siloxanes: Environment tier II assessment" Archived 2020-05-27 at the Wayback Machine Australian Department of Health. Retrieved 2018-12-04.
- ↑ "Candidate List of substances of very high concern for Authorisation – Decamethylcyclopentasiloxane". ECHA. Retrieved 2018-07-18.
- ↑ "Commission Regulation (EU) 2018/35 of 10 January 2018 amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards octamethylcyclotetrasiloxane ('D4') and decamethylcyclopentasiloxane ('D5')". Retrieved 2018-07-18.
External links
- Cyclopentasiloxane in the Consumer Product Information Database
- Dekant, Wolfgang; Klaunig, James E. (2016). "Toxicology of decamethylcyclopentasiloxane (D5)". Regulatory Toxicology and Pharmacology. 74: S67–S76. doi:10.1016/j.yrtph.2015.06.011. PMID 26111607.
