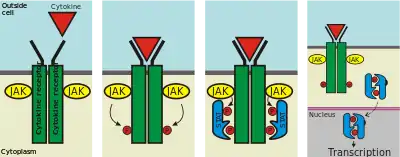
Cytokine receptors are receptors that bind to cytokines.[1]
In recent years, the cytokine receptors have come to demand the attention of more investigators than cytokines themselves, partly because of their remarkable characteristics, and partly because a deficiency of cytokine receptors has now been directly linked to certain debilitating immunodeficiency states. In this regard, and also because the redundancy and pleiotropy of cytokines are a consequence of their homologous receptors, many authorities are now of the opinion that a classification of cytokine receptors would be more clinically and experimentally useful.
Classification
A classification of cytokine receptors based on their three-dimensional structure has been attempted. (Such a classification, though seemingly cumbersome, provides several unique perspectives for attractive pharmacotherapeutic targets.)
- Type I cytokine receptors, whose members have certain conserved motifs in their extracellular amino-acid domain. The IL-2 receptor belongs to this chain, whose γ-chain (common to several other cytokines) deficiency is directly responsible for the x-linked form of Severe Combined Immunodeficiency (X-SCID).
- Type II cytokine receptors, whose members are receptors mainly for interferons.
- Immunoglobulin (Ig) superfamily, which are ubiquitously present throughout several cells and tissues of the vertebrate body
- Tumor necrosis factor receptor family, whose members share a cysteine-rich common extracellular binding domain, and includes several other non-cytokine ligands like receptors, CD40, CD27 and CD30, besides the ligands on which the family is named (TNF).
- Chemokine receptors, two of which acting as binding proteins for HIV (CXCR4 and CCR5). They are G protein coupled receptors.
- TGF-beta receptor family, which are Serine/threonine kinase receptors. Includes the TGF beta receptors
Comparison
| Type | Examples | Structure | Mechanism |
|---|---|---|---|
| type I cytokine receptor | Certain conserved motifs in their extracellular amino-acid domain. Connected to Janus kinase (JAK) family of tyrosine kinases. Many have a FN-III superfamily domain and an immunoglobulin-like fold. | JAK phosphorylate and activate downstream proteins involved in their signal transduction pathways | |
| type II cytokine receptor | |||
| Many members of the immunoglobulin superfamily | Share structural homology with immunoglobulins (antibodies), cell adhesion molecules, and even some cytokine. Includes with the two classes above. | ||
| Tumor necrosis factor receptor family | cysteine-rich common extracellular binding domain | ||
| chemokine receptors |
|
Seven transmembrane helix, rhodopsin-like receptor[2] | G protein-coupled |
| TGF-beta receptor family | Serine/threonine kinase receptors | Dimeric TGFBR2 binds to TGFB and phosphorylates TGFBR1, which phosphorylates the SMADs. See TGF beta signaling pathway. | |
Solubility
Cytokine receptors may be both membrane-bound and soluble. Soluble cytokine receptors are extremely common regulators of cytokine function. Soluble cytokine receptors typically consist of the extracellular portions of membrane-bound receptors. .[3]
See also
References
- ↑ Brooks, Andrew J.; Dehkhoda, Farhad; Kragelund, Birthe B. (2017). "Cytokine Receptors". Principles of Endocrinology and Hormone Action. Springer International Publishing. pp. 1–29. doi:10.1007/978-3-319-27318-1_8-2. ISBN 9783319273181.
- ↑ Arimont A, Sun S, Smit MJ, Leurs R, de Esch IJ, de Graaf C (2017). "Structural Analysis of Chemokine Receptor-Ligand Interactions". J Med Chem. 60 (12): 4735–4779. doi:10.1021/acs.jmedchem.6b01309. PMC 5483895. PMID 28165741.
- ↑ Heaney ML1, Golde DW (1998). "Soluble receptors in human disease". Journal of Leukocyte Biology. 64 (2): 135–146. doi:10.1002/jlb.64.2.135. PMID 9715251. S2CID 34021597.
{{cite journal}}: CS1 maint: numeric names: authors list (link)
External links
- Cytokine-cytokine receptor interaction map from KEGG
- Cytokine+receptors at the U.S. National Library of Medicine Medical Subject Headings (MeSH)