 | |
| Names | |
|---|---|
| Preferred IUPAC name
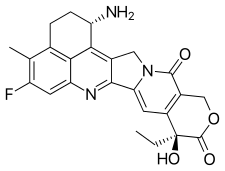
(1S,9S)-1-Amino-9-ethyl-5-fluoro-9-hydroxy-4-methyl-1,2,3,9,12,15-hexahydro-10H,13H-benzo[de]pyrano[3′,4′:6,7]indolizino[1,2-b]quinoline-10,13-dione | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C24H22FN3O4 | |
| Molar mass | 435.455 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Exatecan is a drug which is a structural analog of camptothecin with antineoplastic activity.[1]
A derivative is used in Trastuzumab deruxtecan.
Synthesis
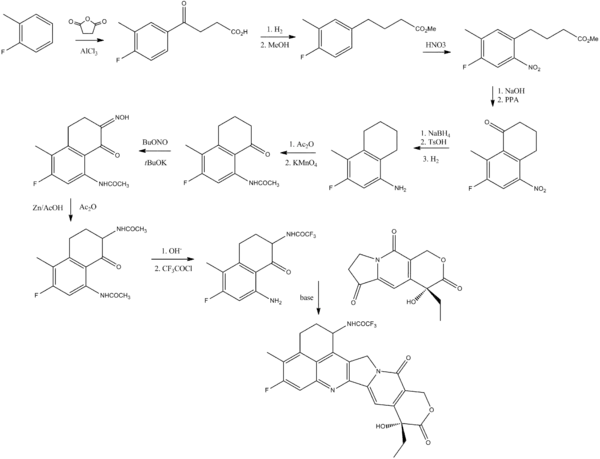
Exatecan synthesis[2]
References
- ↑ Abou-Alfa, GK; Letourneau, R; Harker, G; Modiano, M; Hurwitz, H; Tchekmedyian, NS; Feit, K; Ackerman, J; De Jager, RL; Eckhardt, SG; O'Reilly, EM (20 September 2006). "Randomized Phase III Study of Exatecan and Gemcitabine Compared with Gemcitabine Alone in Untreated Advanced Pancreatic Cancer". Journal of Clinical Oncology. 24 (27): 4441–7. doi:10.1200/JCO.2006.07.0201. PMID 16983112.
- ↑ H. Terasawa, A. Ejima, S. Ohsuki, K. Uoto, U.S. Patent 5,834,476 (1998).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.