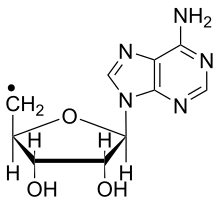
 Structure of the deoxyadenosyl radical | |
| Names | |
|---|---|
| IUPAC name
5′-Deoxyadenosin-5′-yl | |
| Systematic IUPAC name
[(2R,3R,4S,5R)-5-(6-Amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| C10H12N5O3 | |
| Molar mass | 250.238 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
A deoxyadenosyl radical is a free radical that is structurally related to adenosine by removal of a 5′-hydroxy group from adenosine. This radical occurs in nature as a reactive intermediate. It is generated by radical SAM enzymes and by some varieties of vitamin B12.[1] The deoxyadenosyl radical abstracts hydrogen atoms from substrates, causing rearrangements and other post transcriptional modifications required for biosynthesis.[2]
References
- ↑ Jennifer Bridwell-Rabb; Tsehai A. J. Grell; Catherine L. Drennan (2018). "A Rich Man, Poor Man Story of S-Adenosylmethionine and Cobalamin Revisited". Annual Review of Biochemistry. 87: 555–84. doi:10.1146/annurev-biochem-062917-012500. PMID 29925255. S2CID 49354135.
- ↑ Broderick, J. B.; Duffus, B. R.; Duschene, K. S.; Shepard, E. M. (2014). "Radical S-Adenosylmethionine Enzymes". Chemical Reviews. 114 (8): 4229–4317. doi:10.1021/cr4004709. PMC 4002137. PMID 24476342.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.