 | |
.png.webp) | |
| Clinical data | |
|---|---|
| Trade names | Synalar, Iluvien, others |
| AHFS/Drugs.com | Monograph Monograph |
| Pregnancy category |
|
| Routes of administration | Topical, ophthalmic intravitreal injection |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Liver, CYP3A4-mediated |
| Elimination half-life | 1.3 to 1.7 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.607 |
| Chemical and physical data | |
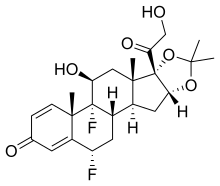
| Formula | C24H30F2O6 |
| Molar mass | 452.495 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Fluocinolone acetonide is a corticosteroid primarily used in dermatology to reduce skin inflammation and relieve itching. It is a synthetic hydrocortisone derivative. The fluorine substitution at position 9 in the steroid nucleus greatly enhances its activity. It was first synthesized in 1959 in the Research Department of Syntex Laboratories S.A. Mexico City.[2] Preparations containing it were first marketed under the name Synalar. A typical dosage strength used in dermatology is 0.01–0.025%. One such cream is sold under the brand name Flucort-N and includes the antibiotic neomycin.
Fluocinolone acetonide was also found to strongly potentiate TGF-β-associated chondrogenesis of bone marrow mesenchymal stem/progenitor cells, by increasing the levels of collagen type II by more than 100 fold compared to the widely used dexamethasone.[3]
Fluocinolone acetonide intravitreal implants have been used to treat non-infectious uveitis. A systematic review could not determine with any confidence whether fluocinolone acetonide implants are superior to standard of care treatment for uveitis.[4] A fluocinolone acetonide intravitreal implant with the brand name Iluvien is sold by biopharmaceutical company Alimera Sciences to treat diabetic macular edema (DME).[5]
It was approved for medical use in 1961.[6]
Classification
Fluocinolone is a group V (0.025%) or group VI (0.01%) corticosteroid under US classification.
Society and culture
Brand names
Yutiq.[7]
References
- ↑ "Regulatory Decision Summary for Iluvien". Drug and Health Product Portal. Health Canada. 23 October 2014.
- ↑ Mills JS, Bowers A, Djerassi C, Ringold HJ (1960). "Steroids CXXXVII. Synthesis of a New Class of Potent Cortical Hormones. 6α,9α-Difluoro-16α-Hydroxyprednisolone and its Acetonide". Journal of the American Chemical Society. 80 (13): 3399–3404. doi:10.1021/ja01498a041.
- ↑ Hara ES, Ono M, Pham HT, Sonoyama W, Kubota S, Takigawa M, et al. (September 2015). "Fluocinolone Acetonide Is a Potent Synergistic Factor of TGF-β3-Associated Chondrogenesis of Bone Marrow-Derived Mesenchymal Stem Cells for Articular Surface Regeneration". Journal of Bone and Mineral Research. 30 (9): 1585–1596. doi:10.1002/jbmr.2502. PMC 5569386. PMID 25753754.
- ↑ Reddy A, Liu SH, Brady CJ, Sieving PC, Palestine AG (August 2023). "Corticosteroid implants for chronic non-infectious uveitis". The Cochrane Database of Systematic Reviews. 2023 (8): CD010469. doi:10.1002/14651858.CD010469.pub4. PMC 10464657. PMID 37642198.
- ↑ "Real-world study shows long-term safety, efficacy of Iluvien in DME". Healio. 2020-07-02. Retrieved 2020-10-28.
- ↑ Fischer J, Ganellin CR, eds. (2006). "Tables of Structural and Functional Analogues: Systemic Hormonal Preparations". Analogue-based Drug Discovery. John Wiley & Sons. p. 485. ISBN 9783527607495.
- ↑ "Yutiq- fluocinolone acetonide implant". DailyMed. 16 October 2023. Retrieved 25 December 2023.