| Knoevenagel condensation | |
|---|---|
| Named after | Emil Knoevenagel |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | knoevenagel-condensation |
| RSC ontology ID | RXNO:0000044 |
In organic chemistry, the Knoevenagel condensation (pronounced [ˈknøːvənaːɡl̩]) reaction is a type of chemical reaction named after German chemist Emil Knoevenagel. It is a modification of the aldol condensation.[1][2]
A Knoevenagel condensation is a nucleophilic addition of an active hydrogen compound to a carbonyl group followed by a dehydration reaction in which a molecule of water is eliminated (hence condensation). The product is often an α,β-unsaturated ketone (a conjugated enone).
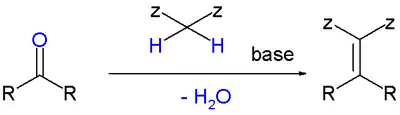
In this reaction the carbonyl group is an aldehyde or a ketone. The catalyst is usually a weakly basic amine. The active hydrogen component has the form[3]
- Z−CH2−Z or Z−CHR−Z for instance diethyl malonate, Meldrum's acid, ethyl acetoacetate or malonic acid, or cyanoacetic acid.[4]
- Z−CHRR', for instance nitromethane.
where Z is an electron withdrawing group. Z must be powerful enough to facilitate deprotonation to the enolate ion even with a mild base. Using a strong base in this reaction would induce self-condensation of the aldehyde or ketone.
The Hantzsch pyridine synthesis, the Gewald reaction and the Feist–Benary furan synthesis all contain a Knoevenagel reaction step. The reaction also led to the discovery of CS gas.
Doebner modification
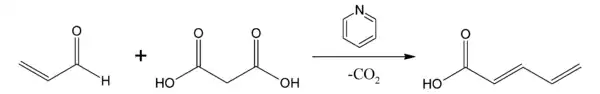
When one of the withdrawing groups on the nucleophile is a carboxylic acid, for example, with malonic acid, the condensation product can undergo a decarboxylation process in a subsequent step. In the so-called Doebner modification[5] the base is pyridine. For example, the reaction product of acrolein and malonic acid in pyridine is trans-2,4-Pentadienoic acid with one carboxylic acid group and not two.[6]
Scope
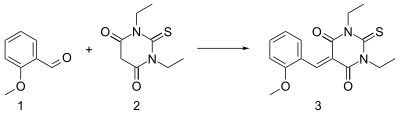
A Knoevenagel condensation is demonstrated in the reaction of 2-methoxybenzaldehyde 1 with the thiobarbituric acid 2 in ethanol using piperidine as a base.[7] The resulting enone 3 is a charge transfer complex molecule.
The Knoevenagel condensation is a key step in the commercial production of the antimalarial drug lumefantrine (a component of Coartem):[8]
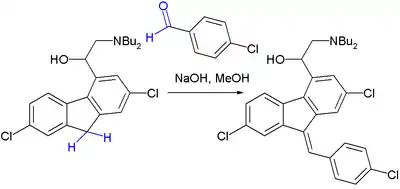
The initial reaction product is a 50:50 mixture of E and Z isomers but because both isomers equilibrate rapidly around their common hydroxyl precursor, the more stable Z-isomer can eventually be obtained.
A multicomponent reaction featuring a Knoevenagel condensation is demonstrated in this MORE synthesis with cyclohexanone, malononitrile and 3-amino-1,2,4-triazole:[9]
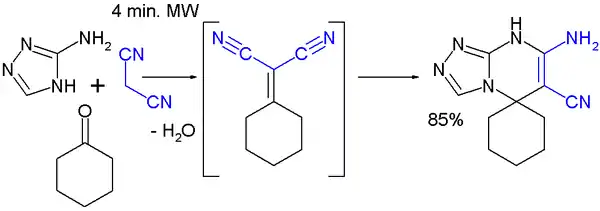
Weiss–Cook reaction
The Weiss–Cook reaction consists in the synthesis of cis-bicyclo[3.3.0]octane-3,7-dione employing an acetonedicarboxylic acid ester and a diacyl (1,2 ketone). The mechanism operates in the same way as the Knoevenagel condensation:[10]
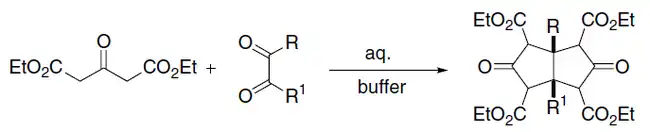
See also
References
- ↑ Jones, G. Org. React. 1967, 15.
- ↑ Emil Knoevenagel (1898). "Condensation von Malonsäure mit aromatischen Aldehyden durch Ammoniak und Amine" [Condensation of malonic acid with aromatic aldehydes via ammonia and amines]. Berichte der deutschen chemischen Gesellschaft. 31 (3): 2596–2619. doi:10.1002/cber.18980310308.
- ↑ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 3rd edition, New York: Wiley, ISBN 9780471854722, OCLC 642506595
- ↑ G. Jones (2004). "The Knoevenagel Condensation". Organic Reactions. pp. 204–599. doi:10.1002/0471264180.or015.02. ISBN 0471264180.
- ↑ O. Doebner (1902). "Ueber die der Sorbinsäure homologen, ungesättigten Säuren mit zwei Doppelbindungen". Berichte der deutschen chemischen Gesellschaft. 35: 1136–36. doi:10.1002/cber.190203501187.
- ↑ Jessup, Peter J.; Petty, C. Bruce; Roos, Jan; Overman, Larry E. (1988). "1-N-Acylamino-1,3-dienes from 2,4-pentadienoic acids by the Curtius rearrangement: benzyl trans-1,3-butadiene-1-carbamate". Organic Syntheses.; Collective Volume, vol. 6, p. 95
- ↑ 1,3-Diethyl-5-(2-methoxybenzylidene)-2-thioxodihydropyrimidine-4,6(1H,5H)-dione Abdullah Mohamed Asiria, Khaled Ahmed Alamrya Abraham F. Jalboutb, Suhong Zhang Molbank 2004, M359 Archived 9 July 2011 at the Wayback Machine publication.
- ↑ An Improved Manufacturing Process for the Antimalaria Drug Coartem. Part II Ulrich Beutler, Peter C. Fuenfschilling, and Andreas Steinkemper Org. Process Res. Dev.; 2007; 11(3) pp. 341–45; (Article) doi:10.1021/op060244p
- ↑ Mild and ecofriendly tandem synthesis of 1,2,4-triazolo[4,3-a]pyrimidines in aqueous medium Arkivoc 2007 (06-2251BP) Anshu Dandia, Pritima Sarawgi, Kapil Arya, and Sarita Khaturia Link
- ↑ Weiss, U.; Edwards, J. M. (1968). "A one-step synthesis of ketonic compounds of the pentalane, [3,3,3]- and [4,3,3]-propellane series". Tetrahedron Letters. 9 (47): 4885. doi:10.1016/S0040-4039(00)72784-5.