| Enteropathic arthropathy | |
|---|---|
| Other names | Enteropathic Arthritis |
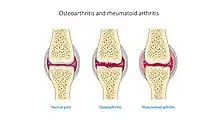 | |
| A comparison of joints in osteoarthritis and rheumatoid arthritis and normal joints | |
| Pronunciation | |
| Specialty | Gastroenterology rheumatology |
| Complications | Chronic arthritis, and toxicity of therapy.[3] |
| Risk factors | Crohn's disease, ulcerative colitis, inflammatory bowel diseases, Whipple's disease, celiac disease, and intestinal bypass surgery.[4] |
| Differential diagnosis | Fibromyalgia, Poncet disease, Reactive arthritis, Bowel–associated dermatosis-arthritis syndrome, Bechet disease, Hypertrophic osteoarthropathy, and SAPHO syndrome.[5] |
Enteropathic arthropathy commonly referred to as enteropathic arthritis, is a type of arthritis linked to Crohn's disease, ulcerative colitis, and chronic inflammatory bowel diseases.[4]
Along with reactive arthritis, psoriatic arthritis, and idiopathic ankylosing spondylitis, this type of arthritis is categorized as a seronegative spondyloarthropathy.[4]
Other gastrointestinal disorders like Whipple's disease, celiac disease, and intestinal bypass surgery for severe obesity can also cause joint involvement. The pathogenesis of arthritis in these conditions is likely influenced by immunologic, genetic, and abnormal bowel permeability factors, though the precise mechanisms are still unknown.[4]
Signs and symptoms
Historically, there have been two main patterns of joint involvement with enteropathic arthritis: axial involvement, which includes sacroiliitis with or without spondylitis resembling idiopathic ankylosing spondylitis, and peripheral arthritis.[4] Additional conditions that may manifest are enthesopathy, tendinitis, and a variety of other conditions such as periostitis, clubbing, and granulomatous lesions of the bone and joint.[6]
Two types of peripheral arthritis have been identified. Pauciarticular arthritis, or type I, usually affects fewer than five major weight-bearing joints. It usually has an asymmetrical pattern and is linked to active bowel disease; monoarthritis isn't uncommon. Both big and small joints are affected, mainly lower limb joints like the metatarsophalangeal joints, ankles, and knees. Shoulder and hip arthritis is less common and is typically linked to spondylitis and sacroiliitis.[7] It does not cause joint deformities, but it is typically migratory, transitory, and recurrent.[8] Joint symptoms, particularly in Crohn's disease, can manifest before bowel symptoms do. In ulcerative colitis, the time of the initial arthritis attack appears to be unrelated to the length of the colitis. Furthermore, a relapse of peripheral arthritis is often associated with a flare-up of the gut symptoms, primarily in ulcerative colitis.[7]
Polyarthritis type II primarily affects the small joints. Rarely does it come before an IBD diagnosis. It usually progresses on its own schedule apart from the bowel illness. Months may pass during an active synovitis episode, and it may recur frequently. Years may pass between periods of exacerbations and remissions.[7]
The spectrum of axial involvement includes true ankylosing spondylitis, which is characterized by classic clinical and radiologic features, as well as asymptomatic sacroiliitis and inflammatory pain in the lower back regardless of the radiological evidence of the condition. Axial involvement may occur years before bowel disease. Inflammatory low back pain, buttocks pain, and chest pain are the main complaints. Typically occurring before the age of 45, inflammatory back pain often begins slowly, is often unilateral, sporadic, and worsens while at rest. It can also be aggravated by coughing or sneezing, worsen in the morning, and cause fatigue. The pain persists for at least three months. Enthesitis of the manubriocostal, costosternal, and costovertebral articulations causes thoracic pain. It worsens with coughing and deep inspirations, and it restricts the expansion of the respiratory system with varying-length episodes. Ankylosing spondylitis has been linked with dactylitis. It is distinguished by tenosynovitis that affects the flexor tendons resulting in inflammatory swelling in one or more fingers or toes. One of the main indicators that the disease is progressing toward generalized ankylosis is the restriction of cervical spine mobility.[7]
Causes
It is unknown what exactly causes the enteropathic arthropathies. Increased permeability from GI tract inflammation can lead to the absorption of antigenic substances, such as bacterial antigens. The musculoskeletal tissues, such as entheses and the synovial membrane, may then localize these arthrogenic antigens, triggering an inflammatory response. Alternatively, by means of molecular mimicry, which involves the host's immune system reacting to these antigens in a cross-reaction with self-antigens present in the synovial membrane and other target organs, an autoimmune response may be triggered.[9]
Risk factors
Depending on the study population as well as the definitions used, systemic disorders such as inflammatory bowel disease (IBD), Crohn's disease (CD), and ulcerative colitis (UC) may be made worse by external manifestations in up to 40% of patients. In IBD, rheumatological manifestations are common and include secondary osteoporosis, secondary hypertrophic osteoarthropathy, axial involvement, peripheral enthesitis, and peripheral arthritis.[7]
Whipple's disease is an uncommon multisystem illness caused by a bacteria called Tropheryma whippelii. Clinical manifestations include dementia, polyarthralgia, low-grade fever, diarrhea, weight loss, lymphadenopathy, and neuropsychiatric symptoms.[10] Arthralgia, as well as arthritis, are the sole signs in 67% of patients, and they may appear years before other symptoms.[11] A case series comprising twenty-five patients with Whipple's disease-related arthropathy revealed that symmetric migratory polyarthritis, primarily affecting the knees, ankles, and wrists, was the most prevalent pattern.[12] Up to 40% of patients have been reported to have axial involvement with lumbar and sacroiliac tenderness.[13] Radiologic signs resembling those of ankylosing spondylitis have also been observed.[14]
Celiac disease is a type of gluten-sensitive enteropathy marked by small intestinal mucosal abnormalities, particularly villous flattening, and atrophy, which leads to malabsorption. Among the numerous clinical signs and symptoms of malabsorption are dermatitis herpetiformis, weight loss, diarrhea, and anemia.[15]First documented in 1982, there is a specific link between celiac disease and inflammatory arthritis.[16] Large joints like the knees, hips, and shoulders are commonly affected by polyarticular symmetrical arthritis, which is the most frequently reported articular pattern.[17] There have also been reports of polyarticular small and large joint arthropathy,[18] oligoarthritis,[19] and monoarthritis.[20] Axial along with peripheral arthritis is linked to celiac disease and can sometimes occur in adults and children[21] before or without bowel symptoms.[22]
In an effort to treat morbid obesity, small intestine bypass surgeries first emerged in the 1950s. The goal was to decrease the absorptive surface of the gut. In 20% to 80% of patients, postoperative arthritis-dermatitis syndrome and several metabolic side effects occur. When the jejunoileal bypass was used in place of the jejunocolonic bypass, similar problems still occurred.[23] Though mono- and oligoarthritis have also been reported, the typical clinical presentation is a migratory non-erosive seronegative polyarthritis affecting the ankles, wrists, shoulders, hands, and fingers.[24] Usually, the arthritis appears two to three years following the surgery.[25] Intestinal bypass arthritis has been linked to the overgrowth of bacteria in the blind loop segment of the bowel, and immunologic involvement appears likely due to the presence of immune complexes in the serum.[26] Reversing the bypass surgery is typically linked to a total and permanent remission of the arthritis, providing more proof of the connection between intestines and joint pathology.[24]
Diagnosis
No pathognomonic finding exists to support a diagnosis of IBD-related arthritis. In the appropriate clinical context, a suspicion of the diagnosis may arise. Radiographs of the pelvis and spine may reveal characteristic features of sacroiliitis and ankylosing spondylitis. The latter is usually bilateral, though reports have indicated a higher incidence of zygapophyseal joint ankylosis and asymmetric sacroiliitis. Although erosive lesions, primarily of the metatarsal joints, are reported, radiographic changes are typically not associated with peripheral joint involvement. Certain characteristics of these lesions differ from those of rheumatoid arthritis, such as the absence of osteoporosis as well as the presence of neighboring bone proliferation. Occasionally, a damaging granulomatous synovitis linked to Crohn's disease may be observed. Radiographically, enteropathies are similar to those observed in other spondyloarthritis.[7]
Treatment
Establishing the efficacy of therapy can be challenging in certain patients due to the episodic nature of their arthropathy. Simple interventions like rest, splints, intra-articular steroid injections, and physical therapy are used to treat many patients.[4]
NSAIDs are usually effective in treating spondyloarthropathy patients; however, they should be used cautiously as they may worsen IBD[27] and have been linked to ulcerations in both the large and small intestines.[28]
Controlling intestinal inflammation is the primary treatment objective for peripheral arthritis, as the disease's activity frequently resembles that of bowel disease. For ulcerative colitis, sulfasalazine has been demonstrated to be beneficial in treating flare-ups as well as the underlying condition;[29] however, the picture for Crohn's disease is less clear. Sulfasalazine has been demonstrated to be beneficial in certain trials for Crohn's disease,[30] but not in others.[31]
The development of targeted biological treatments has had an impact on enteropathic arthritis management. Psoriatic arthritis,[32][33] enthesopathy linked to spondyloarthropathy,[34] and refractory ankylosing spondylitis[35] can all benefit from biological blockade using the TNFa antagonists etanercept and infliximab. Infliximab is also useful in reducing spondyloarthropathy and intestinal inflammation in Crohn's disease patients.[36] Etanercept did not affect bowel disease, but anecdotal evidence points to etanercept's potential benefit in treating spondyloarthritis linked to Crohn's disease, including MRI-demonstrated resolution of spondylitis.[37]
See also
References
- ↑ "How do you pronounce... » Help You to Learn English with Youtube » YThi". YThi. November 6, 2019. Retrieved December 8, 2023.
- ↑ "Definition of ARTHROPATHY". Merriam-Webster. December 7, 2023. Retrieved December 8, 2023.
- ↑ Minerva P (November 15, 2021). "Enteropathic Arthropathies: Practice Essentials, Etiology, Epidemiology". Medscape Reference. Retrieved December 10, 2023.
- 1 2 3 4 5 6 Holden W, Orchard T, Wordsworth P (August 2003). "Enteropathic arthritis". Rheumatic Diseases Clinics of North America. Elsevier BV. 29 (3): 513–30, viii. doi:10.1016/s0889-857x(03)00043-7. PMID 12951865.
- ↑ Shahid Z, Lucke M (July 17, 2023). "Enteropathic Arthritis". StatPearls. StatPearls Publishing. PMID 37603630. Retrieved December 10, 2023.
- ↑ De Keyser F, Elewaut D, De Vos M, De Vlam K, Cuvelier C, Mielants H, Veys EM (November 1998). "Bowel inflammation and the spondyloarthropathies". Rheumatic Diseases Clinics of North America. Elsevier BV. 24 (4): 785–813. doi:10.1016/s0889-857x(05)70042-9. PMID 9891711.
- 1 2 3 4 5 6 Voulgari PV (2011). "Rheumatological manifestations in inflammatory bowel disease". Annals of Gastroenterology. The Hellenic Society of Gastroenterology. 24 (3): 173–180. PMC 3959315. PMID 24713717.
- ↑ Wordsworth P (April 2000). "Arthritis and inflammatory bowel disease". Current Rheumatology Reports. Springer Science and Business Media LLC. 2 (2): 87–88. doi:10.1007/s11926-000-0045-3. PMID 11123044. S2CID 35543053.
- ↑ Minerva P (November 15, 2021). "Enteropathic Arthropathies: Practice Essentials, Etiology, Epidemiology". Medscape Reference. Retrieved December 9, 2023.
- ↑ Kucharz EJ, Kramza J, Grosicka A, Pieczyrak R (April 27, 2021). "Clinical manifestations of Whipple's disease mimicking rheumatic disorders". Reumatologia. Termedia Sp. z.o.o. 59 (2): 104–110. doi:10.5114/reum.2021.105418. PMC 8103404. PMID 33976464.
- ↑ Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P (May 1997). "Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Société Nationale Française de Médecine Interne". Medicine. Ovid Technologies (Wolters Kluwer Health). 76 (3): 170–184. doi:10.1097/00005792-199705000-00003. PMID 9193452.
- ↑ Weiner SR, Utsinger P (February 1986). "Whipple disease". Seminars in Arthritis and Rheumatism. Elsevier BV. 15 (3): 157–167. doi:10.1016/0049-0172(86)90013-2. PMID 2421412.
- ↑ Khan MA (1982). "Axial arthropathy in Whipple's disease". The Journal of Rheumatology. J Rheumatol. 9 (6): 928–929. PMID 6186807.
- ↑ Ayoub WT, Davis DE, Torretti D, Viozzi FJ (1982). "Bone destruction and ankylosis in Whipple's disease". The Journal of Rheumatology. 9 (6): 930–931. PMID 6186808. Retrieved 9 December 2023.
- ↑ Mäki M, Collin P (June 1997). "Coeliac disease". Lancet. Elsevier BV. 349 (9067): 1755–1759. doi:10.1016/s0140-6736(96)70237-4. PMID 9193393. S2CID 40153030.
- ↑ Adelizzi RA, Pecora AA, Chiesa JC (July 1982). "Celiac disease: case report with an associated arthropathy". The American Journal of Gastroenterology. Am J Gastroenterol. 77 (7): 481–485. PMID 7091138.
- ↑ Bourne JT, Kumar P, Huskisson EC, Mageed R, Unsworth DJ, Wojtulewski JA (September 1985). "Arthritis and coeliac disease". Annals of the Rheumatic Diseases. BMJ. 44 (9): 592–598. doi:10.1136/ard.44.9.592. PMC 1001716. PMID 3876079.
- ↑ McDonagh JE, Griffiths ID (December 1992). "Arthritis--a presenting feature of occult coeliac disease". British Journal of Rheumatology. 31 (12): 857–858. doi:10.1093/rheumatology/31.12.857-a. PMID 1458297.
- ↑ Collin P, Korpela M, Hällström O, Viander M, Keyriläinen O, Mäki M (1992). "Rheumatic complaints as a presenting symptom in patients with coeliac disease". Scandinavian Journal of Rheumatology. Informa UK Limited. 21 (1): 20–23. doi:10.3109/03009749209095057. PMID 1570482.
- ↑ Borg AA, Dawes PT, Swan CH, Hothersall TE (January 1994). "Persistent monoarthritis and occult coeliac disease". Postgraduate Medical Journal. Oxford University Press (OUP). 70 (819): 51–53. doi:10.1136/pgmj.70.819.51. PMC 2397588. PMID 8140024.
- ↑ Mäki M, Hallström O, Verronen P, Reunala T, Lähdeaho ML, Holm K, Visakorpi JK (February 1988). "Reticulin antibody, arthritis, and coeliac disease in children". Lancet. Elsevier BV. 1 (8583): 479–480. doi:10.1016/s0140-6736(88)91280-9. PMID 2893907. S2CID 41488212.
- ↑ Chakravarty K, Scott DG (May 1992). "Oligoarthritis--a presenting feature of occult coeliac disease". British Journal of Rheumatology. Oxford University Press (OUP). 31 (5): 349–350. doi:10.1093/rheumatology/31.5.349. PMID 1581779.
- ↑ Shagrin JW, Frame B, Duncan H (September 1971). "Polyarthritis in obese patients with intestinal bypass". Annals of Internal Medicine. American College of Physicians. 75 (3): 377–380. doi:10.7326/0003-4819-75-3-377. PMID 5568149.
- 1 2 Delamere JP, Baddeley RM, Walton KW (October 1983). "Jejuno-ileal bypass arthropathy: its clinical features and associations". Annals of the Rheumatic Diseases. BMJ. 42 (5): 553–557. doi:10.1136/ard.42.5.553. PMC 1001295. PMID 6625703.
- ↑ Clegg DO, Zone JJ, Samuelson CO, Ward JR (April 1985). "Circulating immune complexes containing secretory IgA in jejunoileal bypass disease". Annals of the Rheumatic Diseases. BMJ. 44 (4): 239–244. doi:10.1136/ard.44.4.239. PMC 1001619. PMID 3985690.
- ↑ Utsinger PD (June 1980). "Systemic immune complex disease following intestinal bypass surgery: bypass disease". Journal of the American Academy of Dermatology. Elsevier BV. 2 (6): 488–495. doi:10.1016/s0190-9622(80)80149-6. PMID 6447168.
- ↑ Kaufmann HJ, Taubin HL (October 1987). "Nonsteroidal anti-inflammatory drugs activate quiescent inflammatory bowel disease". Annals of Internal Medicine. American College of Physicians. 107 (4): 513–516. doi:10.7326/0003-4819-107-4-513. PMID 3498419.
- ↑ Bjarnason I, Hayllar J, MacPherson AJ, Russell AS (June 1993). "Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans". Gastroenterology. Elsevier BV. 104 (6): 1832–1847. doi:10.1016/0016-5085(93)90667-2. PMID 8500743.
- ↑ Dissanayake AS, Truelove SC (December 1973). "A controlled therapeutic trial of long-term maintenance treatment of ulcerative colitis with sulphazalazine (Salazopyrin)". Gut. BMJ. 14 (12): 923–926. doi:10.1136/gut.14.12.923. PMC 1412871. PMID 4150435.
- ↑ Van Hees PA, Van Lier HJ, Van Elteren PH, Driessen M, Van Hogezand RA, Ten Velde GP, et al. (May 1981). "Effect of sulphasalazine in patients with active Crohn's disease: a controlled double-blind study". Gut. BMJ. 22 (5): 404–409. doi:10.1136/gut.22.5.404. PMC 1419240. PMID 6114023.
- ↑ Wenckert A, Kristensen M, Eklund AE, Barany F, Jarnum S, Worning H, et al. (1978). "The long-term prophylactic effect of salazosulphapyridine (Salazopyrin) in primarily resected patients with Crohn's disease. A controlled double-blind trial". Scandinavian Journal of Gastroenterology. Informa UK Limited. 13 (2): 161–167. doi:10.3109/00365527809181743. PMID 24891.
- ↑ Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ (July 2000). "Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial". Lancet. Elsevier BV. 356 (9227): 385–390. doi:10.1016/s0140-6736(00)02530-7. PMID 10972371. S2CID 9392760.
- ↑ Antoni C, Manger B (2002). "Infliximab for psoriasis and psoriatic arthritis". Clinical and Experimental Rheumatology. 20 (6 Suppl 28): S122–S125. PMID 12463461. Retrieved 10 December 2023.
- ↑ Marzo-Ortega H, McGonagle D, O'Connor P, Emery P (September 2001). "Efficacy of etanercept in the treatment of the entheseal pathology in resistant spondylarthropathy: a clinical and magnetic resonance imaging study". Arthritis and Rheumatism. 44 (9): 2112–2117. doi:10.1002/1529-0131(200109)44:9<2112::AID-ART363>3.0.CO;2-H. PMID 11592375.
- ↑ Brandt J, Haibel H, Cornely D, Golder W, Gonzalez J, Reddig J, et al. (June 2000). "Successful treatment of active ankylosing spondylitis with the anti-tumor necrosis factor alpha monoclonal antibody infliximab". Arthritis and Rheumatism. 43 (6): 1346–1352. doi:10.1002/1529-0131(200006)43:6<1346::AID-ANR18>3.0.CO;2-E. PMID 10857793.
- ↑ Van den Bosch F, Kruithof E, De Vos M, De Keyser F, Mielants H (November 2000). "Crohn's disease associated with spondyloarthropathy: effect of TNF-alpha blockade with infliximab on articular symptoms". Lancet. Elsevier BV. 356 (9244): 1821–1822. doi:10.1016/s0140-6736(00)03239-6. PMID 11117919. S2CID 1799909.
- ↑ Marzo-Ortega H, McGonagle D, O'Connor P, Emery P (January 2003). "Efficacy of etanercept for treatment of Crohn's related spondyloarthritis but not colitis". Annals of the Rheumatic Diseases. BMJ. 62 (1): 74–76. doi:10.1136/ard.62.1.74. PMC 1754306. PMID 12480676.
Further reading
- Fragoulis GE, Liava C, Daoussis D, Akriviadis E, Garyfallos A, Dimitroulas T (May 2019). "Inflammatory bowel diseases and spondyloarthropathies: From pathogenesis to treatment". World Journal of Gastroenterology. Baishideng Publishing Group Inc. 25 (18): 2162–2176. doi:10.3748/wjg.v25.i18.2162. PMC 6526158. PMID 31143068.
- Barkhodari A, Lee KE, Shen M, Shen B, Yao Q (June 2022). "Inflammatory Bowel Disease: Focus on Enteropathic Arthritis and Therapy". Rheumatology and Immunology Research. Walter de Gruyter GmbH. 3 (2): 69–76. doi:10.2478/rir-2022-0012. PMC 9524814. PMID 36465324.
- Peluso R, Manguso F, Vitiello M, Iervolino S, Di Minno MN (March 2015). "Management of arthropathy in inflammatory bowel diseases". Therapeutic Advances in Chronic Disease. 6 (2): 65–77. doi:10.1177/2040622314563929. PMC 4331233. PMID 25729557.