 | |
| Names | |
|---|---|
| Preferred IUPAC name
(2E,6E)-3,7,11-Trimethyldodeca-2,6,10-trien-1-yl trihydrogen diphosphate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| MeSH | farnesyl+pyrophosphate |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C15H28O7P2 | |
| Molar mass | 382.330 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Farnesyl pyrophosphate (FPP), also known as farnesyl diphosphate (FDP), is an intermediate in the biosynthesis of terpenes and terpenoids such as sterols and carotenoids.[1] It is also used in the synthesis of CoQ (part of the electron transport chain), as well as dehydrodolichol diphosphate (a precursor of dolichol, which transports proteins to the ER lumen for N-glycosylation).
Biosynthesis
Farnesyl pyrophosphate synthase (a prenyl transferase)[2] catalyzes sequential condensation reactions of dimethylallyl pyrophosphate with 2 units of 3-isopentenyl pyrophosphate to form farnesyl pyrophosphate, as is shown in the following two steps:
- Dimethylallyl pyrophosphate reacts with 3-isopentenyl pyrophosphate to form geranyl pyrophosphate:
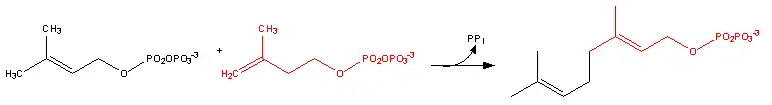
- Geranyl pyrophosphate then reacts with another molecule of 3-isopentenyl pyrophosphate to form farnesyl pyrophosphate
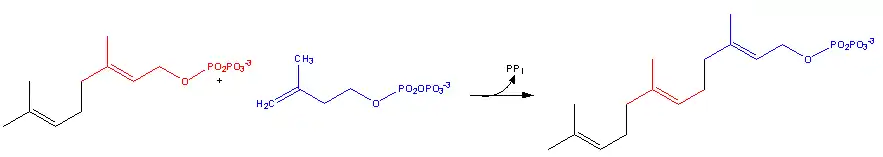
Pharmacology
The above reactions are inhibited by bisphosphonates (used for osteoporosis).[3] Farnesyl pyrophosphate is a selective agonist of TRPV3.[4]
Related compounds
References
- ↑ Davis EM, Croteau R (2000). "Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes". Topics in Current Chemistry. 209: 53–95. doi:10.1007/3-540-48146-X_2. ISBN 978-3-540-66573-1. S2CID 53419212.
- ↑ Kulkarni RS, Pandit SS, Chidley HG, Nagel R, Schmidt A, Gershenzon J, et al. (October 2013). "Characterization of three novel isoprenyl diphosphate synthases from the terpenoid rich mango fruit". Plant Physiology and Biochemistry. 71: 121–131. doi:10.1016/j.plaphy.2013.07.006. PMID 23911730.
- ↑ Russell RG (April 2006). "Bisphosphonates: from bench to bedside". Annals of the New York Academy of Sciences. 1068 (April 2006): 367–401. Bibcode:2006NYASA1068..367R. doi:10.1196/annals.1346.041. PMID 16831938. S2CID 20706956.
- ↑ Bang S, Yoo S, Yang TJ, Cho H, Hwang SW (June 2010). "Farnesyl pyrophosphate is a novel pain-producing molecule via specific activation of TRPV3". The Journal of Biological Chemistry. 285 (25): 19362–71. doi:10.1074/jbc.M109.087742. PMC 2885216. PMID 20395302.