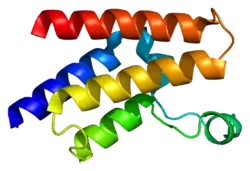
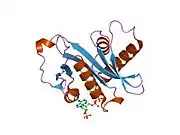
Histone acetyltransferase KAT2A is an enzyme that in humans is encoded by the KAT2A gene.[5][6]
Interactions
GCN5L2 has been shown to interact with:
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000108773 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000020918 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ Candau R, Moore PA, Wang L, Barlev N, Ying CY, Rosen CA, Berger SL (February 1996). "Identification of human proteins functionally conserved with the yeast putative adaptors ADA2 and GCN5". Mol Cell Biol. 16 (2): 593–602. doi:10.1128/mcb.16.2.593. PMC 231038. PMID 8552087.
- ↑ "Entrez Gene: GCN5L2 GCN5 general control of amino-acid synthesis 5-like 2 (yeast)".
- 1 2 3 Martinez E, Palhan VB, Tjernberg A, Lymar ES, Gamper AM, Kundu TK, Chait BT, Roeder RG (October 2001). "Human STAGA complex is a chromatin-acetylating transcription coactivator that interacts with pre-mRNA splicing and DNA damage-binding factors in vivo". Mol. Cell. Biol. 21 (20): 6782–95. doi:10.1128/MCB.21.20.6782-6795.2001. PMC 99856. PMID 11564863.
- 1 2 3 Barlev NA, Poltoratsky V, Owen-Hughes T, Ying C, Liu L, Workman JL, Berger SL (March 1998). "Repression of GCN5 histone acetyltransferase activity via bromodomain-mediated binding and phosphorylation by the Ku-DNA-dependent protein kinase complex". Mol. Cell. Biol. 18 (3): 1349–58. doi:10.1128/mcb.18.3.1349. PMC 108848. PMID 9488450.
- ↑ Wang L, Mizzen C, Ying C, Candau R, Barlev N, Brownell J, Allis CD, Berger SL (January 1997). "Histone acetyltransferase activity is conserved between yeast and human GCN5 and is required for complementation of growth and transcriptional activation". Mol. Cell. Biol. 17 (1): 519–27. doi:10.1128/mcb.17.1.519. PMC 231776. PMID 8972232.
- ↑ Brand M, Moggs JG, Oulad-Abdelghani M, Lejeune F, Dilworth FJ, Stevenin J, Almouzni G, Tora L (June 2001). "UV-damaged DNA-binding protein in the TFTC complex links DNA damage recognition to nucleosome acetylation". EMBO J. 20 (12): 3187–96. doi:10.1093/emboj/20.12.3187. PMC 150203. PMID 11406595.
Further reading
- Berry R, Stevens TJ, Walter NA, Wilcox AS, Rubano T, Hopkins JA, Weber J, Goold R, Soares MB, Sikela JM (1995). "Gene-based sequence-tagged-sites (STSs) as the basis for a human gene map". Nat. Genet. 10 (4): 415–23. doi:10.1038/ng0895-415. PMID 7670491. S2CID 22277955.
- Yang XJ, Ogryzko VV, Nishikawa J, Howard BH, Nakatani Y (1996). "A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A". Nature. 382 (6589): 319–24. Bibcode:1996Natur.382..319Y. doi:10.1038/382319a0. PMID 8684459. S2CID 4328685.
- Wang L, Mizzen C, Ying C, Candau R, Barlev N, Brownell J, Allis CD, Berger SL (1997). "Histone acetyltransferase activity is conserved between yeast and human GCN5 and is required for complementation of growth and transcriptional activation". Mol. Cell. Biol. 17 (1): 519–27. doi:10.1128/mcb.17.1.519. PMC 231776. PMID 8972232.
- Carter KC, Wang L, Shell BK, Zamir I, Berger SL, Moore PA (1997). "The human transcriptional adaptor genes TADA2L and GCN5L2 colocalize to chromosome 17q12-q21 and display a similar tissue expression pattern". Genomics. 40 (3): 497–500. doi:10.1006/geno.1996.4605. PMID 9073520.
- Barlev NA, Poltoratsky V, Owen-Hughes T, Ying C, Liu L, Workman JL, Berger SL (1998). "Repression of GCN5 histone acetyltransferase activity via bromodomain-mediated binding and phosphorylation by the Ku-DNA-dependent protein kinase complex". Mol. Cell. Biol. 18 (3): 1349–58. doi:10.1128/mcb.18.3.1349. PMC 108848. PMID 9488450.
- Smith ER, Belote JM, Schiltz RL, Yang XJ, Moore PA, Berger SL, Nakatani Y, Allis CD (1998). "Cloning of Drosophila GCN5: conserved features among metazoan GCN5 family members". Nucleic Acids Res. 26 (12): 2948–54. doi:10.1093/nar/26.12.2948. PMC 147644. PMID 9611240.
- Randhawa GS, Bell DW, Testa JR, Feinberg AP (1998). "Identification and mapping of human histone acetylation modifier gene homologues". Genomics. 51 (2): 262–9. doi:10.1006/geno.1998.5370. PMID 9722949.
- Xu W, Edmondson DG, Roth SY (1998). "Mammalian GCN5 and P/CAF acetyltransferases have homologous amino-terminal domains important for recognition of nucleosomal substrates". Mol. Cell. Biol. 18 (10): 5659–69. doi:10.1128/MCB.18.10.5659. PMC 109152. PMID 9742083.
- Brand M, Yamamoto K, Staub A, Tora L (1999). "Identification of TATA-binding protein-free TAFII-containing complex subunits suggests a role in nucleosome acetylation and signal transduction". J. Biol. Chem. 274 (26): 18285–9. doi:10.1074/jbc.274.26.18285. PMID 10373431.
- McMahon SB, Wood MA, Cole MD (2000). "The essential cofactor TRRAP recruits the histone acetyltransferase hGCN5 to c-Myc". Mol. Cell. Biol. 20 (2): 556–62. doi:10.1128/MCB.20.2.556-562.2000. PMC 85131. PMID 10611234.
- Kurooka H, Honjo T (2000). "Functional interaction between the mouse notch1 intracellular region and histone acetyltransferases PCAF and GCN5". J. Biol. Chem. 275 (22): 17211–20. doi:10.1074/jbc.M000909200. PMID 10747963.
- Hudson BP, Martinez-Yamout MA, Dyson HJ, Wright PE (2000). "Solution structure and acetyl-lysine binding activity of the GCN5 bromodomain". J. Mol. Biol. 304 (3): 355–70. doi:10.1006/jmbi.2000.4207. PMID 11090279.
- Col E, Caron C, Seigneurin-Berny D, Gracia J, Favier A, Khochbin S (2001). "The histone acetyltransferase, hGCN5, interacts with and acetylates the HIV transactivator, Tat". J. Biol. Chem. 276 (30): 28179–84. doi:10.1074/jbc.M101385200. PMID 11384967.
- Brand M, Moggs JG, Oulad-Abdelghani M, Lejeune F, Dilworth FJ, Stevenin J, Almouzni G, Tora L (2001). "UV-damaged DNA-binding protein in the TFTC complex links DNA damage recognition to nucleosome acetylation". EMBO J. 20 (12): 3187–96. doi:10.1093/emboj/20.12.3187. PMC 150203. PMID 11406595.
- Gangloff YG, Pointud JC, Thuault S, Carré L, Romier C, Muratoglu S, Brand M, Tora L, Couderc JL, Davidson I (2001). "The TFIID components human TAF(II)140 and Drosophila BIP2 (TAF(II)155) are novel metazoan homologues of yeast TAF(II)47 containing a histone fold and a PHD finger". Mol. Cell. Biol. 21 (15): 5109–21. doi:10.1128/MCB.21.15.5109-5121.2001. PMC 87236. PMID 11438666.
- Martinez E, Palhan VB, Tjernberg A, Lymar ES, Gamper AM, Kundu TK, Chait BT, Roeder RG (2001). "Human STAGA complex is a chromatin-acetylating transcription coactivator that interacts with pre-mRNA splicing and DNA damage-binding factors in vivo". Mol. Cell. Biol. 21 (20): 6782–95. doi:10.1128/MCB.21.20.6782-6795.2001. PMC 99856. PMID 11564863.
- Yanagisawa J, Kitagawa H, Yanagida M, Wada O, Ogawa S, Nakagomi M, Oishi H, Yamamoto Y, Nagasawa H, McMahon SB, Cole MD, Tora L, Takahashi N, Kato S (2002). "Nuclear receptor function requires a TFTC-type histone acetyl transferase complex". Mol. Cell. 9 (3): 553–62. doi:10.1016/S1097-2765(02)00478-1. PMID 11931763.
- Brès V, Kiernan R, Emiliani S, Benkirane M (2002). "Tat acetyl-acceptor lysines are important for human immunodeficiency virus type-1 replication". J. Biol. Chem. 277 (25): 22215–21. doi:10.1074/jbc.M201895200. PMID 11956210.
- Col E, Gilquin B, Caron C, Khochbin S (2002). "Tat-controlled protein acetylation". J. Biol. Chem. 277 (40): 37955–60. doi:10.1074/jbc.M206694200. PMID 12154097.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.