Geopolymers are inorganic, typically ceramic, alumino-silicate forming long-range, covalently bonded, non-crystalline (amorphous) networks. Obsidian (volcanic glass) fragments are a component of some geopolymer blends.[1] Commercially produced geopolymers may be used for fire- and heat-resistant coatings and adhesives, medicinal applications, high-temperature ceramics, new binders for fire-resistant fiber composites, toxic and radioactive waste encapsulation and new cements for concrete. The properties and uses of geopolymers are being explored in many scientific and industrial disciplines: modern inorganic chemistry, physical chemistry, colloid chemistry, mineralogy, geology, and in other types of engineering process technologies. The field of geopolymers is a part of polymer science, chemistry and technology that forms one of the major areas of materials science.
Polymers are either organic material, i.e. carbon-based, or inorganic polymer, for example silicon-based. The organic polymers comprise the classes of natural polymers (rubber, cellulose), synthetic organic polymers (textile fibers, plastics, films, elastomers, etc.) and natural biopolymers (biology, medicine, pharmacy). Raw materials used in the synthesis of silicon-based polymers are mainly rock-forming minerals of geological origin, hence the name: geopolymer. Joseph Davidovits coined the term in 1978[2] and created the non profit French scientific institution (Association Loi 1901) Institut Géopolymère (Geopolymer Institute).
According to T.F. Yen[3] geopolymers can be classified into two major groups: pure inorganic geopolymers and organic containing geopolymers, synthetic analogues of naturally occurring macromolecules. In the following presentation, a geopolymer is essentially a mineral chemical compound or mixture of compounds consisting of repeating units, for example silico-oxide (-Si-O-Si-O-), silico-aluminate (-Si-O-Al-O-), ferro-silico-aluminate (-Fe-O-Si-O-Al-O-) or alumino-phosphate (-Al-O-P-O-), created through a process of geopolymerization.[4] This mineral synthesis (geosynthesis) was first presented at an IUPAC symposium in 1976.[5]
The microstructure of geopolymers is essentially temperature dependent: it is X-ray amorphous at room temperature, but evolves into a crystalline matrix at temperatures above 500 °C.[6]
One can distinguish between two synthesis routes: in alkaline media (Na+, K+, Li+, Ca2+, Cs+ and the like); or in acidic media with phosphoric acid, organic carboxylic acids from plant extracts (acetic, citric, oxalic, and humic acids).
In the beginning of 2000s the alkaline route was the most important in terms of research and development and commercial applications and is described below. The acidic route is discussed elsewhere.[7][8]
Definition
In the 1950s, Viktor Glukovsky, of Kiev, USSR, developed concrete materials originally known under the names "soil silicate concretes" and "soil cements",[9] but since the introduction of the geopolymer concept by Joseph Davidovits, 1991, the terminology and definitions of 'geopolymer' have become more diverse and often conflicting. The examples below were taken from 2011 scientific publications, written by scientists with different backgrounds.
Definitions of the term geopolymer[10]
For chemists[11]
- '...Geopolymers consist of a polymeric Si–O–Al framework, similar to zeolites. The main difference to zeolite is geopolymers are amorphous instead of crystalline. The microstructure of geopolymers on a nanometer scale observed by TEM comprises small aluminosilicate clusters with pores dispersed within a highly porous network. The clusters sizes are between 5 and 10 nanometers.'
For geopolymer material chemists[12]
- '...The reaction produces SiO4 and AlO4, tetrahedral frameworks linked by shared oxygens as poly(sialates) or poly(sialate–siloxo) or poly(sialate–disiloxo) depending on the SiO2/Al2O3 ratio in the system. The connection of the tetrahedral frameworks is occurred via long-range covalent bonds. Thus, geopolymer structure is perceived as dense amorphous phase consisting of semi-crystalline 3-D alumino-silicate microstructure.'
For alkali-cement scientists[13]
- '... Geopolymers are framework structures produced by condensation of tetrahedral aluminosilicate units, with alkali metal ions balancing the charge associated with tetrahedral Al. Conventionally, geopolymers are synthesized from a two-part mix, consisting of an alkaline solution (often soluble silicate) and solid aluminosilicate materials. Geopolymerization occurs at ambient or slightly elevated temperature, where the leaching of solid aluminosilicate raw materials in alkaline solutions leads to the transfer of leached species from the solid surfaces into a growing gel phase, followed by nucleation and condensation of the gel phase to form a solid binder.'
For geopolymer ceramic chemists[14]
- '…Although geopolymer is generally X-ray amorphous if cured at standard pressures and temperatures, it will convert into crystalline ceramic phases like leucite or pollucite upon heating.'
For ceramic scientists[15]
- '...Geopolymers are a class of totally inorganic, alumino-silicate based ceramics that are charge balanced by group I oxides. They are rigid gels, which are made under relatively ambient conditions of temperature and pressure into near-net dimension bodies, and which can subsequently be converted to crystalline or glass-ceramic materials.'
Geopolymer synthesis
Ionic coordination vs covalent bonding
In 1937, W. L. Bragg published a method for classifying all kinds of silicates and their crystal structures based on the concept of the ionic theory by Linus Pauling. The fundamental unit is a tetrahedral complex consisting of a small cation such as Si4+, or Al3+ in tetrahedral coordination with four oxygens (Pauling's first rule). Many textbooks explain the geometry of the SiO44− tetrahedron and other mineral structures as determined by the relative sizes of the different ions.
This ionic coordination representation is no longer adapted to the requirements of geopolymer chemistry that is governed by covalent bonding mechanisms. The differences between the ionic concept (coordination) and the covalent bonding are profound. The double tetrahedron structure (coordination) is sharing one oxygen anion O2−, whereas in the Si-O-Si- molecular structure, the covalent bond is achieved through Si and O co-sharing only one electron.[16] This results in stronger bond within the latter structure. The American mineralogist and geochemist G. V. Gibbs and his team studied the polymeric bond Si-O-Si-O and stated in 1982-2000:
The successful modeling of the properties and structures of silica ... lends credence to the statement that a silica polymorph like quartz can be viewed as a giant molecule bound together by essentially the same forces that bind the atoms of the Si-O-Si skeleton into a small siloxane molecule.[17]
The term giant molecule used by G.V. Gibbs is equivalent to the definition of geopolymer and the wording small siloxane molecule describes the actual oligomers of organo-silicon compounds well known as silicone polymer. These siloxane oligomers have the same structure as the silico-aluminate oligomers described below in this article.
Geopolymerization with oligomers
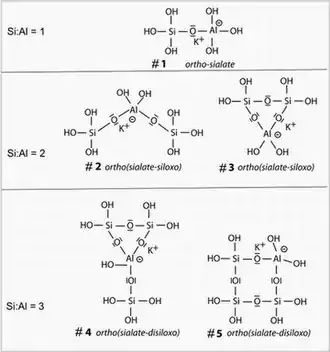
Geopolymerization is the process of combining many small molecules known as oligomers into a covalently bonded network. The geo-chemical syntheses are carried out through oligomers (dimer, trimer, tetramer, pentamer) which provide the actual unit structures of the three-dimensional macromolecular edifice. In 2000, T.W. Swaddle and his team[18] proved the existence of soluble isolated alumino-silicate molecules in solution in relatively high concentrations and high pH. One major improvement in their research was that their study was carried out at very low temperatures, as low as −9 °C. Indeed, it was discovered that the polymerization at room temperature of oligo-sialates was taking place on a time scale of around 100 milliseconds, i.e. 100 to 1000 times faster than the polymerization of ortho-silicate, oligo-siloxo units. At room temperature or higher, the reaction is so fast that it cannot be detected with conventional analytical equipment.
The image shows 5 soluble oligomers of the K-poly(sialate) / poly(sialate-siloxo) species, which are the actual starting units of potassium-based alumino-silicate geopolymerization.
Example of (-Si-O-Al-O-) geopolymerization with metakaolin MK-750 in alkaline medium[19]
It involves four main phases comprising seven chemical reaction steps:
- Alkaline depolymerization of the poly(siloxo) layer of kaolinite;
- Formation of monomeric and oligomeric species, including the "ortho-sialate" (OH)3-Si-O-Al-(OH)3 molecule (#1 in the figure);
- In the presence of waterglass (soluble K-polysiloxonate), one gets the creation of ortho-sialate-disiloxo cyclic structure (e.g. #5 in the figure), whereby the hydroxide is liberated by condensation reactions and can react again;
- Geopolymerization (polycondensation) into higher oligomers and polymeric 3D-networks.
The geopolymerization kinetics for Na-poly(sialate-siloxo) and K-poly(sialate-siloxo) are slightly different respectively. This is probably due to the different dimensions of the Na+ and K+ cations, K+ being bigger than Na+.
Example of zeolitic (Si-O-Al-O-) geopolymerization with fly ash in alkaline medium[20]
It involves 5 main phases
- Nucleation stage in which the aluminosilicates from the fly ash particle dissolve in the alkaline medium (Na+), releasing aluminates and silicates, probably as monomers.
- These monomers inter-react to form dimers, which in turn react with other monomers to form trimers, tetramers and so on.
- When the solution reaches saturation, an aluminum-rich gel (denominated Gel 1) precipitates.
- As the reaction progresses, more Si-O groups from the initial solid source dissolve, increasing the silicon concentration in the medium and gradually raising the proportion of silicon in the zeolite precursor gel (Gel 2).
- Polycondensation into zeolite-like 3D-frameworks.
Geopolymer 3D-frameworks
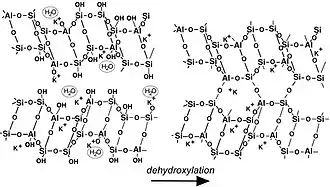
Geopolymerization forms aluminosilicate frameworks that are similar to those of rock-forming minerals. Yet, there are major differences. In 1994, Davidovits[21] presented a theoretical structure for K-poly(sialate-siloxo) (K)-(Si-O-Al-O-Si-O) that was consistent with the NMR spectra. It does not show the presence of water in the structure because he only focused on the relationship between Si, Al, Na, K, atoms. Water is present only at temperatures below 150 °C – 200 °C, essentially in the form of -OH groups, whereas numerous geopolymer industrial and commercial applications work at temperatures above 200 °C, up to 1400 °C, i.e. at temperatures above dehydroxylation. Nevertheless, scientists working on low temperature applications, such as cements and waste management, tried to pinpoint cation hydration and water molecules.[22][23] This model shows an incompletely reacted geopolymer (left in the figure), which involves free Si-OH groups that will later with time or with temperature polycondense with opposed Al-O-K, into Si-O-Al-O sialate bonds. The water released by this reaction either remains in the pores, is associated with the framework similarly to zeolitic water, or can be released and removed. Several 3D-frameworks are described in the book 'Geopolymer Chemistry and Applications'.[24] After dehydroxylation (and dehydration), generally above 250 °C, geopolymers become more and more crystalline (right in the picture) and above 500-1000 °C (depending on the nature of the alkali cation present) crystallise and have X-ray diffraction patterns and framework structures identical to their geological analogues.
Commercial applications
There exist a wide variety of potential and existing applications. Some of the geopolymer applications are still in development whereas others are already industrialized and commercialized. See the incomplete list provided by the Geopolymer Institute.[25] They are listed in three major categories:
Geopolymer resins and binders
- Fire-resistant materials, thermal insulation, foams;
- Low-energy ceramic tiles, refractory items, thermal shock refractories;
- High-tech resin systems, paints, binders and grouts;
- Bio-technologies (materials for medicinal applications);
- Foundry industry (resins), tooling for the manufacture of organic fiber composites;
- Composites for infrastructures repair and strengthening, fire-resistant and heat-resistant high-tech carbon-fiber composites for aircraft interior and automobile;
- Radioactive and toxic waste containment;
Geopolymer cements and concretes
- Low-tech building materials (clay bricks);
- Low-CO2 cements and concretes.
Arts and archaeology
- Decorative stone artifacts, arts and decoration;
- Cultural heritage, archaeology and history of sciences.
Geopolymer resins and binders
The class of geopolymer materials is described by Davidovits to comprise:[26]
- Metakaolin MK-750-based geopolymer binder
- chemical formula (Na,K)-(Si-O-Al-O-Si-O-), ratio Si:Al=2 (range 1.5 to 2.5)
- Silica-based geopolymer binder
- chemical formula (Na,K)-n(Si-O-)-(Si-O-Al-), ratio Si:Al>20 (range 15 to 40).
- Sol-gel-based geopolymer binder (synthetic MK-750)
- chemical formula (Na,K)-(Si-O-Al-O-Si-O-), ratio Si:Al=2
The first geopolymer resin was described in a French patent application filed by J. Davidovits in 1979. The American patent, US 4,349,386, was granted on Sept. 14, 1982 with the title Mineral Polymers and methods of making them. It essentially involved the geopolymerization of alkaline soluble silicate [waterglass or (Na,K)-polysiloxonate] with calcined kaolinitic clay (later coined metakaolin MK-750 to highlight the importance of the temperature of calcination, namely 750 °C in this case). In 1985, Kenneth MacKenzie and his team from New-Zealand, discovered the Al(V) coordination of calcined kaolinite (MK-750), describing a "chemical shift intermediate between tetrahedral and octahedral."[27] This had a great input towards a better understanding of its geopolymeric reactivity.
Since 1979, a variety of resins, binders and grouts were developed by the chemical industry, worldwide.[28]
Potential utilization for geopolymer composites materials
Metakaolin MK-750-based and silica-based geopolymer resins are used to impregnate fibers and fabrics to obtain geopolymer matrix-based fiber composites. These products are fire-resistant; they release no smoke and no toxic fumes. They were tested and recommended by major international institutions such as the American Federal Aviation Administration FAA.[29] FAA selected the carbon-geopolymer composite as the best candidate for the fire-resistant cabin program (1994-1997).[30] Geopolymers are attractive host materials to immobilise nuclear waste due to their high environmental durability and flexibility to compositional changes of waste. They are already used on industrial scale to immobilise difficult radioactive waste streams in Czech Republic and Slovakia.[31][32]
To explore potential applications for geopolymer composite materials, Hsieh and their research team[33] embarked on a study to assess the viability of using metakaolin in combination with varying ratios of potassium hydroxide solution and potassium silicate solution. Additionally, they conducted calcination at temperatures ranging from 1000 to 1300 °C to produce leucite ceramics, a dental prosthesis material. Based on the result, by decreasing the KOH ratio, the water absorption and porosity will decrease due to that when increasing KOH ratio, which may result from the samples cast with higher molarity of KOH having greater absorption due to dependence upon cation type and pH.[34] From the XRD result, when the calcining temperature increased to 1100 °C, the specimens crystallized into a leucite phase[35][36] and might also present cubic leucite and tetragonal leucite.[37]
Fire-resistant material
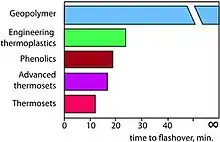
Flashover is a phenomenon unique to compartment fires where incomplete combustion products accumulate at the ceiling and ignite causing total involvement of the compartment materials and signaling the end to human survivability. Consequently, in a compartment fire the time to flashover is the time available for escape and this is the single most important factor in determining the fire hazard of a material or set of materials in a compartment fire. The Federal Aviation Administration has used the time-to-flashover of materials in aircraft cabin tests as the basis for a heat release and heat release rate acceptance criteria for cabin materials for commercial aircraft. The figure shows how the best organic-matrix made of engineering thermoplastics reaches flashover after the 20 minute ignition period and generates appreciable smoke, while the geopolymer-matrix composite will never ignite, reach flashover, or generate any smoke in a compartment fire.
Carbon-geopolymer composite is applied on racing cars around exhaust parts.[38] This technology could be transferred and applied for the mass production of regular automobile parts (corrosion-resistant exhaust pipes and the like) as well as heat shields.[39] A well-known motorcar manufacturer already developed a geopolymer-composite exhaust pipe system.[40]
Geopolymer cements
Production of geopolymer cement requires an aluminosilicate precursor material such as metakaolin or fly ash, a user-friendly alkaline reagent[41] (for example, sodium or potassium soluble silicates with a molar ratio MR SiO2:M2O ≥ 1.65, M being Na or K) and water (See the definition for "user-friendly" reagent below). Room temperature hardening is more readily achieved with the addition of a source of calcium cations, often blast furnace slag.
Portland cement chemistry vs geopolymer chemistry
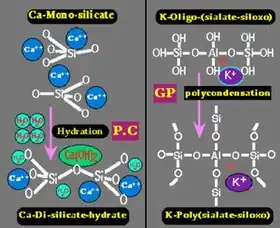
Left: hardening of Portland cement (P.C.) through hydration of calcium silicate into calcium silicate hydrate (C-S-H) and portlandite, Ca(OH)2.
Right: hardening (setting) of geopolymer cement (GP) through poly-condensation of potassium oligo-(sialate-siloxo) into potassium poly(sialate-siloxo) cross linked network.
Geopolymer cement categories
The categories comprise:
- Slag-based geopolymer cement.[42]
- Rock-based geopolymer cement.[43]
- Fly ash-based geopolymer cement
- Ferro-sialate-based geopolymer cement.[48]
Slag-based geopolymer cement
- Components: metakaolin (MK-750) + blast furnace slag + alkali silicate (user-friendly).
- Geopolymeric make-up: Si:Al = 2 in fact solid solution of Si:Al=1, Ca-poly(di-sialate) (anorthite type) + Si:Al = 3 , K-poly(sialate-disiloxo) (orthoclase type) and C-S-H Ca-silicate hydrate.
The first geopolymer cement developed in the 1980s was of the type (K,Na,Ca)-poly(sialate) (or slag-based geopolymer cement) and resulted from the research developments carried out by Joseph Davidovits and J.L. Sawyer at Lone Star Industries, USA and yielded the invention of Pyrament® cement. The American patent application was filed in 1984 and the patent US 4,509,985 was granted on April 9, 1985, with the title 'Early high-strength mineral polymer'. In a study conducted by Ola A. Mayhoub et al.,[49] they explored the replacement of cement with alkali-activated slag in geopolymer cement formulations, with replacement percentages ranging from 35% to 100%. The evaluation of compressive strength under ambient room temperature conditions revealed that the sample with 100% alkali-activated slag achieved a remarkable compressive strength exceeding 42 MPa within a 28-day timeframe.
While the compressive strength of the 100% geopolymer variant did not reach the levels of traditional cement, it demonstrated highly favorable engineering properties. This suggests that geopolymer formulations can offer a compelling and viable alternative in engineering applications.
Sulaem Musaddiq Laskar[50] and team utilized ultrafine ground granulated blast-furnace slag (UGGBS) to formulate geopolymer concrete (GPC) based on UGGBS. Their findings reveal a significant enhancement in mechanical properties compared to traditional cement concrete. In a 28-day evaluation, the compressive strength of GPC incorporating UGGBS was found to be 65.6% higher than that of conventional cement concrete. Similarly, the flexural strength exhibited an impressive 159% increase within the same time frame. Additionally, the versatility of GPC is highlighted, as it can effectively serve as a jacketing material for reinforced concrete (RC) structural components.[51][52][53] Notably, GPC achieves approximately 89% of its 28-day strength in just 3 days, indicating its quick-setting properties and practicality in various construction scenarios. In summary, the results emphasize the favorable mechanical properties of GPC, showcasing its potential as a superior alternative to traditional cement concrete in construction applications.
Rock-based geopolymer cement
The replacement of a certain amount of MK-750 with selected volcanic tuffs yields geopolymer cement with better properties and less CO2 emission than the simple slag-based geopolymer cement.
- Manufacture components: metakaolin MK-750, blast furnace slag, volcanic tuffs (calcined or not calcined), mine tailings and alkali silicate (user-friendly).
- Geopolymeric make-up: Si:Al = 3, in fact solid solution of Si:Al=1 Ca-poly(di-sialate) (anorthite type) + Si:Al = 3-5 (Na,K)-poly(silate-multisiloxo) and C-S-H Ca-silicate hydrate.
Fly ash-based geopolymer cements
Later on, in 1997, building on the works conducted on slag-based geopolymeric cements, on the one hand and on the synthesis of zeolites from fly ashes on the other hand, Silverstrim et al.[54] and van Jaarsveld and van Deventer[55] developed geopolymeric fly ash-based cements. Silverstrim et al. US Patent 5,601,643 was titled 'Fly ash cementitious material and method of making a product'. Numerous researchers have pioneered the development of cementless concrete as a means to mitigate CO2 emissions. Their innovative mix designs not only curtail carbon dioxide exhaust but also allow for the attainment of optimal compressive strength in alkali-activated concrete that incorporates fly ash.[56][57][58] Ryu and colleagues conducted an investigation focused on the complete removal of cement, employing 100% fly ash as the sole binder for alkali-activated concrete.[59]
Analysis of Energy-Dispersive X-ray Spectroscopy (EDS) results unveiled the prominent presence of Si and Al components, predominantly originating from the fly ash. In addition to Si and Al, the EDS analysis detected the presence of sodium (Na) from the alkali activator. This sodium component can be attributed to the formation of reaction products via the condensation process brought about by geopolymerization. This condensation process promotes the agglomeration of reaction products, facilitating strength development through synergistic interactions between fly ash particles.
CO2 emissions during manufacture
According to the Australian concrete expert B. V. Rangan, the growing worldwide demand for concrete is a great opportunity for the development of geopolymer cements of all types, with their much lower tally of carbon dioxide CO2.[60] In 2021, a life cycle assessment study performed by researchers from the University of New South Wales (UNSW Sydney), Australia, confirmed that geopolymer mortars establish compressive strength and flexural strength that are adequate for construction applications and present sustainability benefits in Global Warming Potential, which suggests them to be potential substitutions for Ordinary Portland Cement. However, the industrial waste treatment (i.e., preparation of fly ash) depletes water bodies and the sodium silicate induces significant environmental burdens during its manufacture, becoming the key factor to enhance the geopolymer’s sustainability.[61]
The need for standards
In June 2012, the institution ASTM International organized a symposium on Geopolymer Binder Systems. The introduction to the symposium states: When performance specifications for Portland cement were written, non-portland binders were uncommon...New binders such as geopolymers are being increasingly researched, marketed as specialty products, and explored for use in structural concrete. This symposium is intended to provide an opportunity for ASTM to consider whether the existing cement standards provide, on the one hand, an effective framework for further exploration of geopolymer binders and, on the other hand, reliable protection for users of these materials.
The existing Portland cement standards are not adapted to geopolymer cements. They must be created by an ad hoc committee. Yet, to do so, requires also the presence of standard geopolymer cements. Presently, every expert is presenting his own recipe based on local raw materials (wastes, by-products or extracted). There is a need for selecting the right geopolymer cement category. The 2012 State of the Geopolymer R&D,[62] suggested to select two categories, namely:
- type 2 slag/fly ash-based geopolymer cement: fly ashes are available in the major emerging countries;
- ferro-sialate-based geopolymer cement: this geological iron-rich raw material is present in all countries throughout the globe, and;
- the appropriate user-friendly geopolymeric reagent.
Geopolymer applications to arts and archaeology
Because geopolymer artifacts look like natural stone, several artists started to cast in silicone rubber molds replications of their sculptures. For example, in the 1980s, the French artist Georges Grimal worked on several geopolymer castable stone formulations.[63]
Egyptian pyramid stones
With respect to archaeological applications, in the mid-1980s, Joseph Davidovits presented his first analytical results carried out on genuine pyramid stones. He claimed that the ancient Egyptians knew how to generate a geopolymeric reaction in the making of a re-agglomerated limestone blocks.[64] The Ukrainian scientist G.V. Glukhovsky endorsed Davidovits' research in his keynote paper to the First Intern. Conf. on Alkaline Cements and Concretes, Kiev, Ukraine, 1994.[65] Later on, several materials scientists and physicists took over these archaeological studies and are publishing their results, essentially on pyramid stones.[66][67][68][69]
Roman cements
From the digging of ancient Roman ruins, one knows that approximately 95% of the concretes and mortars constituting the Roman buildings consist of a very simple lime cement, which hardened slowly through the precipitating action of carbon dioxide CO2, from the atmosphere and formation of calcium silicate hydrate (C-S-H). This is a very weak to medium good material that was used essentially in the making of foundations and in buildings for the populace.
But for the building of their "ouvrages d’art", especially works related to water storage (cisterns, aqueducts), the Roman architects did not hesitate to use more sophisticated and expensive ingredients. These outstanding Roman cements are based on the calcic activation of ceramic aggregates (in Latin testa, analogue to our modern metakaolin MK-750) and alkali rich volcanic tuffs (cretoni, zeolitic pozzolan), respectively with lime. MAS-NMR Spectroscopy investigations were carried out on these high-tech Roman cements dating to the 2nd century AD. They show their geopolymeric make-up.[70]
See also
References
- ↑ Kozhukova, N.I.; Chizhov, R.V.; Zhervovsky, I.V.; Strokova, V.V. (2016). Structure Formation of Geopolymer Perlite Binder Vs. Type of Alkali Activating Agent, International Journal of Pharmacy & Technology, vol. 8, iss. no. 3, pp. 15,339.
- ↑ An article published by the Commission of the European Communities in 1982, outlines the reasons why the generic term geopolymer was chosen for this new chemistry. See: J. Davidovits, The Need to Create a New Technical Language For the Transfer of Basic Scientific Information, in Transfer and Exploitation of Scientific and Technical Information, Proceedings of the symposium, Luxemburg, 10–12 June 1981, pp. 316-320. It is available as a pdf-file and may be downloaded from the European Parliament Bookshop. Go to < https://publications.europa.eu/en/publication-detail/-/publication/02a1db8b-3873-46d7-9e72-a6e02660e154 > and click on 'download'.
- ↑ Kim, D.; Lai, H.T.; Chilingar, G.V.; Yen T.F. (2006), Geopolymer formation and its unique properties, Environ. Geol, 51[1], 103–111.
- ↑ "What is a geopolymer? Introduction – Geopolymer Institute".
- ↑ Pdf-file #20 Milestone paper IUPAC 76 at https://www.geopolymer.org/category/library/technical-papers
- ↑ Zoulgami, M; Lucas-Girot, A.; Michaud, V.; Briard, P.; Gaudé, J. and Oudadesse, H. (2002); Synthesis and physico-chemical characterization of a polysialate-hydroxyapatite composite for potential biomedical application, Eur. Phys. J. AP, 19, 173-179. See also: Kriven, W.M.; Bell, J.; Gordon, M. (2003), Microstructure and Microchemistry of Fully-Reacted Geopolymers and Geopolymer Matrix Composites, Ceramic Transactions, 153, 227–250; Perera, D.S. and Trautman R.L. (2005), Geopolymers with the Potential for Use as Refractory Castables, Advances in Technology of Materials and Materials Processing, 7[2], 187–190.
- ↑ Wagh, A.S. (2004), Chemically Bonded Phosphate Ceramics – A Novel Class of Geopolymers, Proceedings of the 106th Ann. Mtg. of the American Ceramic Society, Indianapolis. See also, Chapter 13, Phosphate-based Geopolymers, in J. Davidovits' book Geopolymer Chemistry and Applications.
- ↑ Perera, D.S., Hanna, J.V., Davis, J., Blackford, M.G., Latella, B.A., Sasaki, Y. and Vance E.R. (2008), Relative strengths of phosphoric acid-reacted and alkali-reacted metakaolin materials, J. Mater. Sci., 43, 6562–6566. See also, Cao, D.; Su, D.; Lu, B. and Yang Y. (2005), Synthesis and structure characterization of geopolymeric material based on metakaolinite and phosphoric acid, Journal Chinese Ceramic Society, 33, 1385–89.
- ↑ Gluchovskij V.D.:"Gruntosilikaty" Gosstrojizdat Kiev 1959, Patent USSR 245 627 (1967), Patent USSR 449894 (Patent appl. 1958, granted 1974).
- ↑ See, Discussion at the Geopolymer Camp 2012, video Geopolymer definition in Wikipedia at "Geopolymer Institute » GP Camp 2012". Archived from the original on 2013-04-15. Retrieved 2013-01-18..
- ↑ Huang, Yi and Han, Minfang (2011) (China University of Mining and Technology, Beijing), The influence of α-Al2O3 addition on microstructure, mechanical and formaldehyde adsorption properties of fly ash-based geopolymer products, Journal of Hazardous Materials, 193, 90–94
- ↑ Pimraksaa, K.; Chindaprasirt, P.; Rungchet, A.; Sagoe-Crentsil, K. and Sato, T. (2011) (Department of Industrial Chemistry, Chiang Mai University, Thailand; CSIRO, Melbourne, Australia; Tohoku University, Sendai, Japan), Lightweight geopolymer made of highly porous siliceous materials with various Na2O/Al2O3 and SiO2/Al2O3 ratios, Materials Science and Engineering A, 528, 6616–6623.
- ↑ Feng, Dingwu; Provis, John L. and van Deventer, Jannie S. J. (2012) (University of Melbourne, Australia), Thermal Activation of Albite for the Synthesis of One-Part Mix Geopolymers, J. Am. Ceram. Soc., 95 [2] 565–572.
- ↑ Peigang He, Dechang Jia, Meirong Wang, Yu Zhou, (2011) (Harbin Institute of Technology, Harbin, PR China:), Thermal evolution and crystallization kinetics of potassium-based geopolymer, Ceramics International, 37, 59–63.
- ↑ Bell, Jonathan L.; Driemeyer, Patrick E. and Kriven, Waltraud M. (2009) (University of Illinois, USA), Formation of Ceramics from Metakaolin-Based Geopolymers. Part II: K-Based Geopolymer, J. Am. Ceram. Soc., 92 [3], 607-615.
- ↑ See the figure at https://www.geopolymer.org/science/about-geopolymerization
- ↑ Gibbs, G.V.; Hill, F.C.; Boisen Jr, M.B. and Downs R.T., (2000), Molecules as a Basis for Modeling the Force Field of Silica, Chapter 6 in Structure and Imperfections in Amorphous and Crystalline Silicon Dioxide, Edited by R. A. B. Devine, J.-P. Duraud and E. Dooryhee, John Wiley & Sons Ltd
- ↑ North, M.R. and Swaddle, T.W. (2000). Kinetics of Silicate Exchange in Alkaline Aluminosilicate Solutions, Inorg. Chem., 39, 2661–2665.
- ↑ see at https://www.geopolymer.org/science/about-geopolymerization
- ↑ Duxson, P.; Fernández-Jiménez, A.; Provis, J.l.; Lukey, G.C; Palomo, A. and Van Deventer, J.S.J., (2007), Geopolymer technology: the current state of the art, J. Mat. Sci., 42 (9) 2917–2933.
- ↑ Davidovits, J., (1994), Geopolymers: Man-Made Rock Geosynthesis and the Resulting Development of Very Early High Strength Cement, J. Materials Education, 16 (2&3), 91–139.
- ↑ Barbosa, V.F.F; MacKenzie, K.J.D. and Thaumaturgo, C., (2000), Synthesis and characterization of materials based on inorganic polymers of alumina and silica: sodium polysialate polymers, Intern. Journal of Inorganic Materials, 2, pp. 309–317.
- ↑ Rowles, M.R. (2004), The Structural Nature of Aluminosilicate Inorganic Polymers: a Macro to Nanoscale Study, PhD Thesis, Curtin University of Technology, Perth, Australia.
- ↑ See: Structural frameworks and chemical mechanisms, in Davidovits' book Geopolymer Chemistry and Applications, Sections 8.6-8.7.
- ↑ see at https://www.geopolymer.org/about/business-fellows Archived 2019-09-25 at the Wayback Machine
- ↑ see the Chapters 8, 11, 20 in J. Davidovits' book Geopolymer Chemistry and Applications.
- ↑ Meinhold, R. H.; MacKenzie, K. J. D.; Brown, I. W. M. (1985). "Thermal reactions of kaolinite studied by solid state 27-Al and 29-Si NMR". Journal of Materials Science Letters. 4 (2): 163–166. doi:10.1007/BF00728065. ISSN 0261-8028. S2CID 96064063.
- ↑ see the updates in the Keynotes State of Geopolymer R&D, 2009, 2010, 2011, and 2012 at https://www.geopolymer.org/camp
- ↑ The FAA research project, 1994-1997 involved the collaboration between the research teams of: – FAA Fire Department, Atlantic City, USA; – Rutgers University of New Jersey, USA; – Cordi-Géopolymère laboratory, Saint-Quentin, France. A picture of geopolymer composite testing by FAA (Oil Burner Test of Fireproof composite) can be downloaded at https://www.fire.tc.faa.gov/Research/TargetAreas
- ↑ Lyon, R.E.; Foden, A.J.; Balaguru, P.N.; Davidovits, J. and Davidovics, M. (1997), Properties of Geopolymer Matrix-Carbon Fiber Composites, Fire and Materials, 21, 67–73.
- ↑ R.O. Abdel Rahman, R.Z. Rahimov, N.R. Rahimova, M.I. Ojovan. Cementitious materials for nuclear waste immobilization. ISBN 978-1-118-51200-5, Wiley, Chichester 232 p., (2015)
- ↑ Almkvist, L.; Bai, S.; Bastiaens, W.; Cau-dit-Coumes, C.; Glasser, F.; Govaert, J. (2013). "The Behaviour of Cementitious Materials in Long-Term Storage and Disposal of Radioactive Waste | IAEA-TECDOC-1701, IAEA, 61 p., Vienna (2013)". iaea.org. Retrieved 21 February 2021.
- ↑ Hsieh, Yi-Che; Lee, Wei-Hao; Liao, Pin-Hsun (2021). "Using Geopolymer Technology on Synthesizing Leucite Ceramics". Polymers. 13 (21): 3621. doi:10.3390/polym13213621. PMC 8587864. PMID 34771177.
- ↑ Rehman, Sardar Kashif Ur; Imtiaz, Lahiba; Aslam, Fahid; Khan, Muhammad Khizar; Haseeb, Muhammad; Javed, Muhammad Faisal; Alyousef, Rayed; Alabduljabbar, Hisham (2020). "Experimental Investigation of NaOH and KOH Mixture in SCBA-Based Geopolymer Cement Composite". Materials. 13 (15): 3437. Bibcode:2020Mate...13.3437R. doi:10.3390/ma13153437.
- ↑ Bell, Jonathan L.; Driemeyer, Patrick E.; Kriven, Waltraud M. (2009). "Formation of Ceramics from Metakaolin-Based Geopolymers. Part II: K-Based Geopolymer". Journal of the American Ceramic Society. 92 (3): 607–615. doi:10.1111/j.1551-2916.2008.02922.x.
- ↑ Xie, Ning; Bell, Jonathan. L.; Kriven, Waltraud M. (2010). "Fabrication of Structural Leucite Glass–Ceramics from Potassium-Based Geopolymer Precursors". Journal of the American Ceramic Society. 93 (9): 2644–2649. doi:10.1111/j.1551-2916.2010.03794.x.
- ↑ 黄, 丽婷; 刘, 洋.; 彭, 诚.; 吴, 建青 (2017). "立方相白榴石的合成与稳定". 硅酸盐学报. 45 (7): 948–954. doi:10.14062/j.issn.0454-5648.2017.07.09.
- ↑ Davidovics, M.; Bruno, M. and Davidovits, J. (1999), Past and Present Experience on the Use of Carbon-Geopolymer Composite in Formula One and CART Racing Cars, Geopolymer ’99 Proceedings, 141–142.
- ↑ Davidovits, J. (2002), 30 Years of Successes and Failures in Geopolymer Applications, Market Trends and Potential Breakthroughs, Geopolymer 2002 Conference, Oct. 28-29, Melbourne, Australia. Download the pdf-file #15 at https://www.geopolymer.org/category/library/technical-papers.
- ↑ See the PCT patent application publication WO 2004/106705 filed by Porsche AG, 2004.
- ↑ See the examples at the Geopolymer Institute page https://www.geopolymer.org/applications/geopolymer-cement
- ↑ Davidovits, J. and Sawyer, J.L., (1985), Early high-strength mineral polymer, US Patent 4,509,985, 1985, filed February 22, 1984. The first commercial geopolymer cement was coined Pyrament 2000™ designed for repair and patching operations.
- ↑ Gimeno, D.; Davidovits, J.; Marini, C.; Rocher, P.; Tocco, S.; Cara, S.; Diaz, N.; Segura, C. and Sistu, G. (2003), Development of silicate-based cement from glassy alkaline volcanic rocks: interpretation of preliminary data related to chemical- mineralogical composition of geologic raw materials. Paper in Spanish, Bol. Soc. Esp. Cerám. Vidrio, 42, 69–78. [Results from the European Research Project GEOCISTEM (1997), Cost Effective Geopolymeric Cements For Innocuous Stabilisation of Toxic Elements, Final Technical Report, April 30, 1997, Brussels, Project funded by the European Commission, Brite-Euram BE-7355-93, Jan. 1, 1994 to Feb. 28, 1997].
- ↑ Palomo, A.; Grutzeck, M.W. and Blanco, M.T. (1999), Alkali-activated fly ashes: a cement for the future, Cement Concrete Res, 29, 1323–1329.
- ↑ GEOASH (2004–2007), The GEOASH project was carried out with a financial grant from the Research Fund for Coal and Steel of the European Community, contract number RFC-CR-04005. It involves: Antenucci D., ISSeP, Liège, Belgium; Nugteren H.and Butselaar- Orthlieb V., Delft University of Technology, Delft, The Netherlands; Davidovits J., Cordi-Géopolymère Sarl, Saint-Quentin, France; Fernández-Pereira C. and Luna Y., University of Seville, School of Industrial Engineering, Sevilla, Spain; Izquierdo and M., Querol X., CSIC, Institute of Earth Sciences Jaume Almera, Barcelona, Spain.
- ↑ Izquierdo, M.; Querol, X.; Davidovits, J.; Antenucci, D.; Nugteren, H. and Fernández-Pereira, C., (2009), Coal fly ash-based geopolymers: microstructure and metal leaching, Journal of Hazardous Materials, 166, 561–566.
- ↑ See: Chapter 12 in J. Davidovits' book Geopolymer Chemistry and Applications.
- ↑ Davidovits, J. et al., Geopolymer cement of the Calcium-Ferroaluminium silicate polymer type and production process, PCT patent publication WO 2012/056125.
- ↑ Mayhoub, Ola A.; Nasr, El-Sayed A.R.; Ali, Yehia; Kohail, Mohamed (2021). "Properties of slag based geopolymer reactive powder concrete". Ain Shams Engineering Journal. 12: 99–105. doi:10.1016/j.asej.2020.08.013.
- ↑ Laskar, Sulaem Musaddiq; Talukdar, Sudip (2021). "Slag-Based Geopolymer Concrete as Reinforced Concrete Jacketing Agent". Journal of Materials in Civil Engineering. 33 (7). doi:10.1061/(ASCE)MT.1943-5533.0003780. S2CID 236232062.
- ↑ Sarker, Prabir Kumar (2011). "Bond strength of reinforcing steel embedded in fly ash-based geopolymer concrete". Materials and Structures. 44 (5): 1021–1030. doi:10.1617/s11527-010-9683-8. S2CID 136700644.
- ↑ Sirju, K.; Sharma, A. K. (2001). "Strengthening of reinforced concrete members under compression and bending". Proceedings of the Institution of Civil Engineers - Structures and Buildings. 146 (2): 227–231. doi:10.1680/stbu.2001.146.2.227.
- ↑ Teng, Susanto; Lim, Tze Yang Darren; Sabet Divsholi, Bahador (2013). "Durability and mechanical properties of high strength concrete incorporating ultra fine Ground Granulated Blast-furnace Slag". Construction and Building Materials. 40: 875–881. doi:10.1016/j.conbuildmat.2012.11.052.
- ↑ Silverstrim, T.; Rostami, H.; Larralde, J.C and Samadi-Maybodi, A. (1997), Fly ash cementitious material and method of making a product, US Patent 5,601,643.
- ↑ Van Jaarsveld, J.G.S., van Deventer, J.S.J. and Lorenzen L. (1997), The potential use of geopolymeric materials to immobilize toxic metals: Part I. Theory and Applications, Minerals Engineering, 10 (7), 659–669.
- ↑ https://www.geopolymer.org/wp-content/uploads/curtin-flyash-GP-concrete-report.pdf
- ↑ Geopolymer Concrete with Fly Ash. UWM Center for By-Products Utlilization. 2010. pp. 1493–1504. hdl:20.500.11937/3540. ISBN 9781450714907.
- ↑ https://www.geopolymer.org/wp-content/uploads/curtin_flyash_GC-2.pdf
- ↑ Ryu, Gum Sung; Lee, Young Bok; Koh, Kyung Taek; Chung, Young Soo (2013). "The mechanical properties of fly ash-based geopolymer concrete with alkaline activators". Construction and Building Materials. 47: 409–418. doi:10.1016/j.conbuildmat.2013.05.069.
- ↑ Rangan, B.V., (2008), Low-Calcium Fly Ash-Based Geopolymer Concrete, Chapter 26 in Concrete Construction Engineering Handbook, Editor-in-Chief E.G. Nawy, Second Edition, CRC Press, New York.
- ↑ Tang, W.X.; Pignatta, G.; Sepasgozar, S.M.E. (2021). "Life-Cycle Assessment of Fly Ash and Cenosphere-Based Geopolymer Material". Sustainability. 13 (20): 11167. doi:10.3390/su132011167.
- ↑ See the video at "Geopolymer Institute » GP Camp 2012". Archived from the original on 2013-04-15. Retrieved 2013-01-18.
- ↑ See Potential utilizations in art and decoration, at https://www.geopolymer.org/applications/potential-utilizations-in-art-and-decoration; a pdf article #19 Dramatized sculptures with geopolymers at https://www.geopolymer.org/category/library/technical-papers/
- ↑ Davidovits, J. (1986), X-Rays Analysis and X-Rays Diffraction of Casing Stones from the Pyramids of Egypt, and the Limestone of the Associated Quarries; pp. 511–20 in Science in Egyptology Symposia, Edited by R. A. David, Manchester University Press, Manchester, U.K. (Pdf-file #A in the Geopolymer Institute Library, Archaeological Papers); see also: Davidovits J., (1987), Ancient and modern concretes: what is the real difference? Concrete International: Des. Constr, 9 [12], 23–29. See also: Davidovits, J. and Morris, M., (1988), The Pyramids: An Enigma Solved. Hippocrene Books, New York, 1988.
- ↑ G.V Glukhovsky passed away before the conference. His keynote paper titled: Ancient, Modern and Future Concretes, is included in the Proceedings of the First Intern. Conf. on Alkaline Cements and Concretes, pp. 1-9, Kiev, Ukraine, 1994.
- ↑ Demortier, G. (2004), PIXE, PIGE and NMR study of the masonry of the pyramid of Cheops at Giza, Nuclear Instruments and Methods, Physics Research B, 226, 98–109.
- ↑ Barsoum, M.W.; Ganguly, A. and Hug, G. (2006), Microstructural Evidence of Reconstituted Limestone Blocks in the Great Pyramids of Egypt, J. Am. Ceram. Soc. 89[12], 3788–3796.
- ↑ MacKenzie, Kenneth J.D.; Smith, Mark E.; Wong, Alan; Hanna, John V.; Barry, Bernard and Barsoum, Michel W. (2011), Were the casing stones of Senefru's Bent Pyramid in Dahshour cast or carved? Multinuclear NMR evidence, Materials Letters 65, 350–352.
- ↑ Túnyi, I. and El-hemaly, I. A. (2012), Paleomagnetic investigation of the great egyptian pyramids, Europhysics News 43/6, 28-31.
- ↑ As part of the European research project GEOCISTEM [33], Davidovits J. and Davidovits F. sampled archaeological mortars and concretes dating back to the 2nd century AD and later, in Rome and Ostia, Italy. They selected two series of artifacts: Opus Signinum in Rome, Opus Caementicum / Testacaeum: mortars and concretes (carbunculus), in Ostia. Partly published in Geopolymer ’99 Proceedings, 283-295 and in Davidovits' book, Geopolymer Chemistry and Applications, Section 17.4. See also the NMR spectra at: https://www.geopolymer.org/applications/archaeological-analogues-roman-cements
Bibliography
- Geopolymer Chemistry and Applications, Joseph Davidovits, Institut Géopolymère, Saint-Quentin, France, 2008, ISBN 9782951482050 (3rd ed., 2011). In Chinese: National Defense Industry Press, Beijing, ISBN 9787118074215, 2012.
- Geopolymers Structure, processing, properties and industrial applications, John L. Provis and Jannie S. J. van Deventer, Woodhead Publishing, 2009, ISBN 9781845694494.
External links
- Geopolymer Institute: https://www.geopolymer.org/
- Geopolymer Alliance: https://web.archive.org/web/20130409024601/http://geopolymers.com.au/