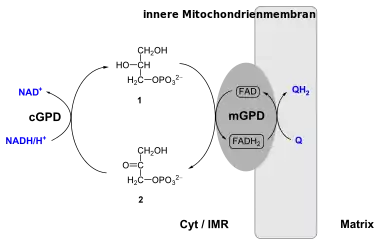
The glycerol-3-phosphate shuttle is a mechanism used in skeletal muscle and the brain[1] that regenerates NAD+ from NADH, a by-product of glycolysis. The NADH generated during glycolysis is found in the cytoplasm and must be transported into the mitochondria to enter the oxidative phosphorylation pathway. However, the inner mitochondrial membrane is impermeable to NADH and NAD+ and does not contain a transport system for these electron carriers. Either the glycerol-3-phosphate shuttle pathway or the malate-aspartate shuttle pathway, depending on the tissue of the organism, must be taken to transport cytoplasmic NADH into the mitochondria.[2] The shuttle consists of the sequential activity of two proteins; Cytoplasmic glycerol-3-phosphate dehydrogenase (cGPD) transfers an electron pair from NADH to dihydroxyacetone phosphate (DHAP), forming glycerol-3-phosphate (G3P) and regenerating NAD+ needed to generate energy via glycolysis.[3] The other protein, mitochondrial glycerol-3-phosphate dehydrogenase (mGPD) catalyzes the oxidation of G3P by FAD, regenerating DHAP in the cytosol and forming FADH2 in the mitochondrial matrix.[4] In mammals, its activity in transporting reducing equivalents across the mitochondrial membrane is considered secondary to the malate-aspartate shuttle.
History
The glycerol phosphate shuttle was first characterized as a major route of mitochondrial hydride transport in the flight muscles of blow flies.[5][6] It was initially believed that the system would be inactive in mammals due to the predominance of lactate dehydrogenase activity over Glycerol-3-phosphate dehydrogenase 1 (GPD1)[5][7] until high GPD1 and GPD2 activity were demonstrated in mammalian brown adipose tissue and pancreatic ß-islets.[8][9][10][11]
Reaction
In this shuttle, the enzyme called cytoplasmic glycerol-3-phosphate dehydrogenase 1 (GPD1 or cGPD) converts dihydroxyacetone phosphate (2) to glycerol 3-phosphate (1) by oxidizing one molecule of NADH to NAD+ as in the following reaction:
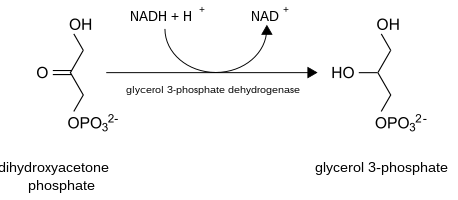
Glycerol-3-phosphate is converted back to dihydroxyacetone phosphate by an inner membrane-bound mitochondrial glycerol-3-phosphate dehydrogenase 2 (GPD2 or mGPD), this time reducing one molecule of enzyme-bound flavin adenine dinucleotide (FAD) to FADH2. FADH2 then reduces coenzyme Q (ubiquinone to ubiquinol) whose electrons enter into oxidative phosphorylation.[12] This reaction is irreversible.[13] These electrons bypass Complex I of the electron transport chain, making the glycerol-3-phosphate shuttle less energetically efficient compared to oxidation of NADH by Complex I.[14]
See also
References
- ↑ Blanco, Antonio; Blanco, Gustavo (2017-01-01), Blanco, Antonio; Blanco, Gustavo (eds.), "Chapter 9 - Biological Oxidations: Bioenergetics", Medical Biochemistry, Academic Press, pp. 177–204, doi:10.1016/b978-0-12-803550-4.00009-4, ISBN 978-0-12-803550-4, retrieved 2023-05-14
- ↑ Bhagavan, N. V. (2002-01-01), Bhagavan, N. V. (ed.), "CHAPTER 14 - Electron Transport and Oxidative Phosphorylation", Medical Biochemistry (Fourth Edition), San Diego: Academic Press, pp. 247–274, doi:10.1016/b978-012095440-7/50016-0, ISBN 978-0-12-095440-7, retrieved 2023-05-14
- ↑ "GPD1 glycerol-3-phosphate dehydrogenase 1 [Homo sapiens (human)] – Gene – NCBI". www.ncbi.nlm.nih.gov. Retrieved 2021-05-03.
- ↑ "GPD2 glycerol-3-phosphate dehydrogenase 2 [Homo sapiens (human)] – Gene – NCBI". www.ncbi.nlm.nih.gov. Retrieved 2021-05-03.
- 1 2 Estabrook RW, Sacktor B (October 1958). "alpha-Glycerophosphate oxidase of flight muscle mitochondria". The Journal of Biological Chemistry. 233 (4): 1014–9. doi:10.1016/S0021-9258(18)64696-4. PMID 13587533.
- ↑ Sacktor B, Dick A (October 1962). "Pathways of hydrogen transport in the oxidation of extramitochondrial reduced diphosphopyridine nucleotide in flight muscle". The Journal of Biological Chemistry. 237 (10): 3259–63. doi:10.1016/S0021-9258(18)50156-3. PMID 13975951.
- ↑ Boxer GE, Shonk CE (January 1960). "Low levels of soluble DPN-linked alpha glycerophosphate dehydrogenase in tumors". Cancer Research. 20: 85–91. PMID 13803504.
- ↑ Ohkawa KI, Vogt MT, Farber E (May 1969). "Unusually high mitochondrial alpha glycerophosphate dehydrogenase activity in rat brown adipose tissue". The Journal of Cell Biology. 41 (2): 441–9. doi:10.1083/jcb.41.2.441. PMC 2107766. PMID 5783866.
- ↑ Houstek J, Cannon B, Lindberg O (1975). "Glycerol-3-phosphate shuttle and its function in intermediate metabolism of hamster brown-adipose tissue". European Journal of Biochemistry. 54 (1): 11–18. doi:10.1111/j.1432-1033.1975.tb04107.x. PMID 168075.
- ↑ Ratner PL, Fisher M, Burkart D, Cook JR, Kozak LP (April 1981). "The role of mRNA levels and cellular localization in controlling sn-glycerol-3-phosphate dehydrogenase expression in tissues of the mouse". The Journal of Biological Chemistry. 256 (7): 3576–9. doi:10.1016/S0021-9258(19)69647-X. PMID 6782104.
- ↑ Koza RA, Kozak UC, Brown LJ, Leiter EH, MacDonald MJ, Kozak LP (December 1996). "Sequence and tissue-dependent RNA expression of mouse FAD-linked glycerol-3-phosphate dehydrogenase". Archives of Biochemistry and Biophysics. 336 (1): 97–104. doi:10.1006/abbi.1996.0536. PMID 8951039.
- ↑ Stryer, Lubert; Berg, Jeremy Mark; Tymoczko, John L. (2007). Biochemistry. San Francisco: W. H. Freeman. ISBN 978-0-7167-8724-2. Archived from the original on 2007-05-18.
- ↑ Shen W, Wei Y, Dauk M, et al. (February 2006). "Involvement of a glycerol-3-phosphate dehydrogenase in modulating the NADH/NAD+ ratio provides evidence of a mitochondrial glycerol-3-phosphate shuttle in Arabidopsis". Plant Cell. 18 (2): 422–41. doi:10.1105/tpc.105.039750. PMC 1356549. PMID 16415206.
- ↑ Mráček, Tomáš; Drahota, Zdeněk; Houštěk, Josef (2013-03-01). "The function and the role of the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian tissues". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1827 (3): 401–410. doi:10.1016/j.bbabio.2012.11.014. ISSN 0005-2728. PMID 23220394.
External links
- http://chemistry.elmhurst.edu/vchembook/601glycolysissum.html (describes the shuttle in the context of glycolysis)