 | |
| Names | |
|---|---|
| IUPAC name
Iodogold | |
| Systematic IUPAC name
Gold(I) iodide | |
| Other names
Gold monoiodide Aurous iodide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.584 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| AuI | |
| Molar mass | 323.871 g/mol |
| Appearance | Yellowish to greenish-yellow powder |
| Density | 8.25 g/cm3[1] |
| −91.0·10−6 cm3/mol | |
| Structure | |
| tetragonal, Pearson symbol tP8, Z = 4 | |
| P42/ncm (No. 138)[1] | |
a = 0.435, b = 0.435, c = 1.373 nm | |
| Hazards | |
| GHS labelling:[2] | |
 | |
| Warning | |
| H315, H319, H335 | |
| P302+P352, P305+P351+P338 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Gold monoiodide is the inorganic compound of gold and iodine with the formula AuI. It can be synthesized by dissolving gold powder in an aqueous solution of iodine and potassium iodide.[3] With Lewis bases, AuI reacts to give numerous complexes.[4]
Preparation
Gold monoiodide can be obtained by reacting a tetrachloridoauric acid solution with furthermore potassium iodide. It is also possible to produce it by reacting gold and iodine in a protective atmosphere at around 390 °C.[5]
Properties
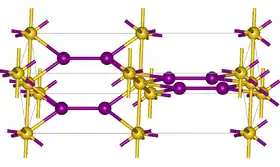
Gold monoiodide is a yellow, crystalline powder that gradually decomposes upon contact with water, humidity or light. It has a tetragonal crystal structure with the space group P42/ncm (space group no. 138), a = 4.359 Å, c = 13.711 Å.[5]
References
- 1 2 Jagodzinski H. (1959). "Die Kristallstruktur des AuJ". Z. Kristallogr. 112 (1–6): 80–87. Bibcode:1959ZK....112...80J. doi:10.1524/zkri.1959.112.1-6.80. S2CID 96721760.
- ↑ Sigma-Aldrich 398411 (13-12-2021)
- ↑ Wilfling, Marion; Klinkhammer, Karl W. (2010). "Gold(I)-Mediated Silicon-Silicon Bond Metathesis at Room Temperature". Angewandte Chemie International Edition. 49 (18): 3219–3223. doi:10.1002/anie.200905950. PMID 20349479.
- ↑ Tang, Zhongjia; Litvinchuk, A. P.; Lee, Hye-G.; Guloy, Arnold M. (1 September 1998). "Crystal Structure and Vibrational Spectra of a New Viologen Gold(I) Iodide". Inorganic Chemistry. 37 (19): 4752–4753. doi:10.1021/ic980141q. PMID 11670634.
- 1 2 Handbuch der präparativen anorganischen Chemie. 2 (3., umgearb. Aufl ed.). Stuttgart: Enke. 1978. ISBN 978-3-432-87813-3.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.