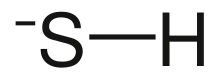 | |
 | |
| Names | |
|---|---|
| IUPAC name
Hydrosulfide | |
| Systematic IUPAC name
Sulfanide (rarely used, not common) | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| 24766 | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| HS− | |
| Molar mass | 33.07 g·mol−1 |
| Conjugate acid | Hydrogen sulfide |
| Conjugate base | Sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Bisulfide (or bisulphide in British English) is an inorganic anion with the chemical formula HS− (also written as SH−). It contributes no color to bisulfide salts, and its salts may have a distinctive putrid smell. It is a strong base. Bisulfide solutions are corrosive and attack the skin.
It is an important chemical reagent and an industrial chemical, mainly used in paper pulp industry (Kraft process), textiles, synthetic flavors, coloring brasses, and iron control.
Properties
A variety of salts are known, including sodium hydrosulfide and potassium hydrosulfide. Ammonium hydrosulfide, a component of "stink bombs" has not been isolated as a pure solid. Some compounds described as salts of the sulfide dianion contain primarily hydrosulfide. For example, the hydrated form of sodium sulfide, nominally with the formula Na2S · 9 H2O, is better described as NaSH · NaOH · 8 H2O.

Aqueous bisulfide absorbs light at around 230 nm in the UV–visible spectrum.[1] Using this approach, bisulfide has been detected in the ocean[2][3] and in sewage.[4] Bisulfide should not be confused with the disulfide dianion, S2−2, or −S–S−.
Basicity
The bisulfide anion can accept a proton:
-
(1)
Because of its affinity to accept a proton (H+), bisulfide has a basic character. In aqueous solution, it has a corresponding pKa value of 6.9. Its conjugate acid is hydrogen sulfide (H2S). However, bisulfide's basicity stems from its behavior as an Arrhenius base. A solution containing spectator-only counter ions, has a basic pH according to the following acid-base reaction:
-
(2)
Chemical reactions
Upon treatment with an acid, bisulfide converts to hydrogen sulfide. With strong acids, it can be doubly protonated to give H
3S+
. Oxidation of bisulfide gives sulfate. When strongly heated, bisulfide salts decompose to produce sulfide salts and hydrogen sulfide.
-
2 HS− → H2S + S2−
(3)
Biochemistry
At physiological pH, hydrogen sulfide is usually fully ionized to bisulfide (HS−). Therefore, in biochemical settings, "hydrogen sulfide" is often used to mean, bisulfide. Hydrosulfide has been identified as the third gasotransmitter along with nitric oxide and carbon monoxide.[5]
Other derivatives
SH− is a soft anionic ligand that forms complexes with most metal ions. Examples include [Au(SH)2]− and (C5H5)2Ti(SH)2, derived from gold(I) chloride and titanocene dichloride, respectively.[6]
Safety
Bisulfide salts are corrosive, strongly alkaline and release toxic hydrogen sulfide upon acidification.
See also
References
- ↑ Goldhaber, M.B.; Kaplan, I.R. (1975), "Apparent dissociation constants of hydrogen sulfide in chloride solutions", Marine Chemistry, 3 (1): 83–104, doi:10.1016/0304-4203(75)90016-X
- ↑ Johnson, K.S.; Coletti, L.S. (2001), "In situ ultraviolet spectrophotometry for high resolution and long-term monitoring of nitrate, bromide and bisulfide in the ocean.", Deep-Sea Research, 49 (7): 1291–1305, Bibcode:2002DSRI...49.1291J, doi:10.1016/s0967-0637(02)00020-1
- ↑ Guenther, E.A.; Johnson, K.S.; Coale, K.H. (2001), "Direct ultraviolet spectrophotometric determination of total sulfide and iodide in natural waters", Analytical Chemistry, 73 (14): 3481–3487, doi:10.1021/ac0013812, PMID 11476251
- ↑ Sutherland-Stacey, L.; Corrie, S.; Neethling, A.; Johnson, I.; Gutierrez, O.; Dexter, R.; Yuan, Z.; Keller, J.; Hamilton, G. (2007), "Continuous measurement of dissolved sulfide in sewer systems", Water Science and Technology
- ↑ J. W. Pavlik, B. C. Noll, A. G. Oliver, C. E. Schulz, W. R. Scheidt, “Hydrosulfide (HS−) Coordination in Iron Porphyrinates”, Inorganic Chemistry, 2010, vol. 49(3), 1017-1026.
- ↑ Peruzzini, M.; de los Rios, I. & Romerosa, A. (2001), "Coordination Chemistry of Transition Metals with Hydrogen Chalcogenide and Hydrogen Chalcogenido Ligands", Progress in Inorganic Chemistry, 49: 169–543, doi:10.1002/9780470166512.ch3, ISBN 978-0-470-16651-2