The Ii antigen system is a human blood group system based upon a gene on chromosome 6 and consisting of the I antigen and the i antigen.[1] The I antigen is normally present on the cell membrane of red blood cells in all adults, while the i antigen is present in fetuses and newborns.[2]
I and i antigens
Adult red blood cells express I antigen abundantly.[3] Developing fetuses and newborns express i antigen until around 13-20 months after birth, when I antigen starts to be expressed instead.[3] Like ABH antigens, which make up the ABO blood group, I and i antigens are not restricted to the red blood cell membrane, but are found on most human cells and in body fluids such as saliva.[1]
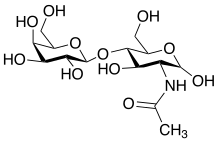
The I and i antigens are carbohydrate structures composed of repeating units of N-acetyllactosamine (LacNAc), and are located on the interior of structures carrying ABH and Lewis antigens.[1][3] LacNAc repeats are made by the enzymes B3GNT1 and B4GALT1.[4] The i antigen is made of linear repeats, while the structure of the I antigen is branched.[3] Unlike most other blood groups, the two antigens are not encoded by different alleles; rather, I-branching enzyme converts i antigen to I antigen by adding branches.[5][6] The gene encoding I-branching enzyme is located on chromosome 6.[6]
Clinical significance
The function of I and i antigens are unknown but may be related to hematopoiesis, the production of blood.[6] The rapid conversion from i to I antigens after birth suggests that I antigen plays an important role in adult red blood cells.[3] The presence of the linear i antigen in fetuses, rather than the branched I antigen, may have developed as an evolutionary mechanism to prevent ABO hemolytic disease of the fetus and newborn.[1] Enhanced expression of i antigen is associated with conditions involving stress hematopoiesis such as leukemia and sickle cell disease.[7]
Transient autoantibodies against I antigen are common, especially after infection by Mycoplasma pneumoniae, and are rarely significant except in cold agglutinin disease.[1] Transient antibodies against i antigen are common after infectious mononucleosis and are also not clinically significant.[1] Antibodies which recognize both I and i antigens are termed anti-j antibodies.[1]
Cold agglutinin disease
The autoantibodies involved in cold agglutinin disease are usually against I antigen.[8] The antibodies are usually IgM (kappa subtype), unlike transient autoantibodies which are generally IgG.[1] Cold-reactive IgM antibodies (cold agglutinins) bind to I antigen on red blood cells, and unlike IgG, are able to cause agglutination of red blood cells and activate complement to cause hemolysis, leading to anemia.[1][8]
Adult i phenotype
Rarely, individuals have the i antigen on their red blood cells into adulthood, known as the adult i phenotype.[1] This is due to the presence of a mutation in the GCNT2 gene which encodes the I-branching enzyme.[1][3] These individuals have alloantibodies against the I antigen, though these are typically cold agglutinins and are unlikely to cause transfusion reactions.[2][9]
The adult i phenotype is associated with congenital cataracts, most markedly in Japanese and Taiwanese people and least markedly in Caucasian people.[1][6] Cataracts occur when i antigen rather than I antigen is present on the epithelium of the lens, due to a mutation in the form of the I-branching enzyme which is expressed in lens epithelium, IGNTB.[10]
The adult i phenotype is inherited in a recessive manner.[1]
History
The I antigen was first described in 1956 and the i antigen was discovered in 1960.[1] I and i were the first discovered antigens which change significantly during human development.[4] The letter I was chosen to reflect the "individuality" of a person studied who lacked the I antigen.[6]
Other species
A similar blood group system with a developmental change resembling the Ii system (with human neonatal cells expressing i antigen and adult cells expressing I antigen) has been observed in most primates, including chimpanzees and monkeys.[1] This is not seen in non-primates: cats, dogs, or guinea pigs.[1]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Daniels G (2013-01-28). "I and i Antigens, and Cold Agglutination". Human Blood Groups. Oxford, UK: Wiley-Blackwell. pp. 469–484. doi:10.1002/9781118493595.ch25. ISBN 978-1-118-49359-5.
- 1 2 Castillo B, Dasgupta A, Klein K, Tint H, Wahed A (2018). "Red cell antigens and antibody". Transfusion Medicine for Pathologists. Elsevier. pp. 69–112. doi:10.1016/b978-0-12-814313-1.00005-8. ISBN 978-0-12-814313-1.
- 1 2 3 4 5 6 Yu LC, Lin M (November 2011). "Molecular genetics of the blood group I system and the regulation of I antigen expression during erythropoiesis and granulopoiesis" (PDF). Current Opinion in Hematology. 18 (6): 421–6. doi:10.1097/MOH.0b013e32834baae9. PMID 21912254. S2CID 205827249.
- 1 2 "OMIM Entry - # 110800 - BLOOD GROUP, I SYSTEM; Ii". www.omim.org. Retrieved 2021-01-31.
- ↑ Pourazar A (January 2007). "Red cell antigens: Structure and function". Asian Journal of Transfusion Science. 1 (1): 24–32. doi:10.4103/0973-6247.28069. PMC 3168130. PMID 21938229.
- 1 2 3 4 5 Reid ME (2020). "The gene encoding the I blood group antigen: review of an I for an eye" (PDF). Immunohematology. 20 (4): 249–52. doi:10.21307/immunohematology-2019-458. PMID 15679458. S2CID 44662081.
- ↑ Reid ME, Lomas-Francis C, Olsson ML (2012). "Ii Blood Group Collection". The Blood Group Antigen Facts Book. Elsevier. pp. 651–653. doi:10.1016/b978-0-12-415849-8.00037-5. ISBN 978-0-12-415849-8.
{{cite book}}:|work=ignored (help) - 1 2 Michalak SS, Olewicz-Gawlik A, Rupa-Matysek J, Wolny-Rokicka E, Nowakowska E, Gil L (November 2020). "Autoimmune hemolytic anemia: current knowledge and perspectives". Immunity & Ageing. 17 (1): 38. doi:10.1186/s12979-020-00208-7. PMC 7677104. PMID 33292368.
- ↑ Poole J, Daniels G (January 2007). "Blood group antibodies and their significance in transfusion medicine". Transfusion Medicine Reviews. 21 (1): 58–71. doi:10.1016/j.tmrv.2006.08.003. PMID 17174221.
- ↑ "OMIM Entry - * 600429 - GLUCOSAMINYL (N-ACETYL) TRANSFERASE 2, I-BRANCHING ENZYME; GCNT2". www.omim.org. Retrieved 2021-01-31.