 | |
| Names | |
|---|---|
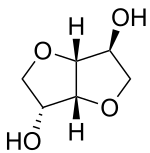
| Preferred IUPAC name
(3R,3aR,6S,6aR)-Hexahydrofuro[3,2-b]furan-3,6-diol | |
| Other names
D-Isosorbide; 1,4:3,6-Dianhydro-D-sorbitol; 1,4-Dianhydrosorbitol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.010.449 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H10O4 | |
| Molar mass | 146.142 g·mol−1 |
| Appearance | Highly hygroscopic white flakes |
| Density | 1.30 at 25 °C |
| Melting point | 62.5 to 63 °C (144.5 to 145.4 °F; 335.6 to 336.1 K) |
| Boiling point | 160 °C (320 °F; 433 K) at 10 mmHg |
| in water (>850 g/L), alcohols and ketones | |
| Pharmacology | |
| None | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
| Clinical data | |
|---|---|
| Trade names | Ismotic, Isobide, others |
| Identifiers | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.010.449 |
Isosorbide is a bicyclic chemical compound from the group of diols and the oxygen-containing heterocycles, containing two fused furan rings. The starting material for isosorbide is D-sorbitol, which is obtained by catalytic hydrogenation of D-glucose, which is in turn produced by hydrolysis of starch. Isosorbide is discussed as a plant-based platform chemical from which biodegradable derivatives of various functionality can be obtained.
In 2020, it was the 114th most commonly prescribed medication in the United States, with more than 5 million prescriptions.[1][2]
Production
Hydrogenation of glucose gives sorbitol. Isosorbide is obtained by acid-catalyzed dehydration of D-sorbitol which yields the monocyclic furanoid sorbitan,[3] which in turn forms by further dehydration the bicyclic furofuran derivative isosorbide.[4]
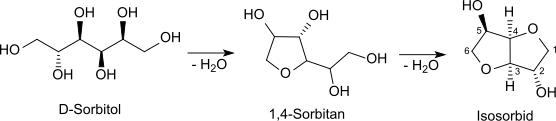
The reaction gives about 70 to 80% isosorbide in addition to 30 to 20% of undesirable by-products which have to be removed costly by distillation, recrystallization from alcohols, recrystallization from the melt,[5] by a combination of these methods or by deposition from the vapor phase.[6] A high purity product (> 99.8%[6]) is essential for the use of a monomer when uncoloured, high molecular weight polymers shall be obtained.
Properties
Isosorbide is a white, crystalline, highly hydrophilic solid. The two secondary hydroxy groups in the V-shaped bicyclic system possess different orientations leading to different chemical reactivities. This allows a selective monoderivatization of isosorbide. The hydroxy group in 5-position is endo oriented and forms a hydrogen bond with the oxygen atom in the adjacent furan ring. This makes the hydroxy group in 5-position more nucleophilic and more reactive than the exo oriented hydroxy group in 2-position; however, it is more shielded from the attack of sterically demanding reactants.[7]
Safety
With an LD50 value of 25.8 g·kg−1 (rat, oral[8]), isosorbide is similarly nontoxic as D-glucose (also with an LD50 of 25.8 g·kg−1, rat, oral[9]) and is classified by the Food and Drug Administration FDA as GRAS ("generally recognized as safe").[10]
Use
Isosorbid
Because of its pronounced hygroscopicity, isosorbide is used as a humectant and in medicine as an osmotic diuretic (for the treatment of hydrocephalus) and acute angle-closure glaucoma.[11] The two secondary hydroxy groups make isosorbide a versatile platform chemical accessible from renewable resources. As a diol, isosorbide can be mono- or biderivatized using the standard methods of organic chemistry, such as nitration, esterification, etherification, tosylation, etc., and converted into compounds with interesting properties or into monomeric units for novel polymers.[3]
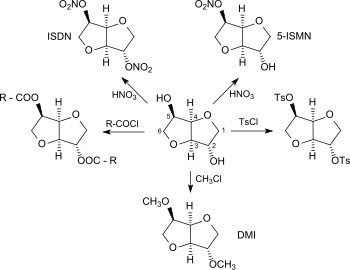
Isosorbide nitrates
By nitration of isosorbide with concentrated nitric acid, 2,5-isosorbide dinitrate (ISDN) can be obtained. 2,5-isosorbide dinitrate is suitable (just like its major metabolite 5-isosorbide mononitrate, ISMN[12]) for the treatment of angina pectoris due to its vasodilator effect.[13]
Isosorbide esters
Esterification of isosorbide with fatty acids gives access to isosorbide monoesters, which are useful as detergents in household cleaners, dishwashing detergents, and cosmetic preparations, because of their properties as surfactant.[14] The likewise readily available isosorbide diesters[15] are used as dispersants for pigments, preservatives, polymer stabilizers, as emulsifiers for cosmetics and as plasticizers for vinyl polymers (in particular polyvinyl chloride, PVC). Isosorbide dioctanoate[16] is a diester of isosorbide and octanoic acid (obtained from palm oil, for example) and therefore made entirely from bio-based building blocks and has been used as Polysorb(R) ID 37 by Roquette Frères for some time as a particularly non-toxic product.[17]
Isosorbide ethers
Isosorbide ethers (and in particular the simplest representative, 2,5-dimethylisosorbide, abbreviated DMI), are increasingly used as a renewable solvent for cosmetic and pharmaceutical preparations,[18] as an electrolyte additive for lithium-ion accumulators[19] [18] and as a fuel additive for diesel.[20]
Isosorbide phosphates
Phosphoric acid derivatives of isosorbide are explored as an environmentally friendly alternative to halogen-containing flame retardants. So far, 1,2,5,6,9,10-hexabromocyclododecane (HBCD) has been widely used as a flame retardant in extruded polystyrene foam (XPS) in the construction and insulation sector, but it was as SVHC (substance of very high concern) banned from manufacturing and application in May 2013. Phosphorus-based isosorbide compounds, such as isosorbide bis (diphenyl phosphate) [ISTP] are considered as a replacement.
.svg.png.webp)
ISTP is readily accessible by transesterification of isosorbide with triphenyl phosphate in the presence of potassium carbonate at 150 °C. The isosorbide-bis-diphenyl phosphate obtained in 88% yield as a yellowish oil contains about 20% dimers.[21] The high decomposition temperature of ISTP allows a use in XPS, although the high softening effect is a drawback. The flame retardancy is particularly pronounced in the presence of sulfur-containing synergists such as bis(diphenylphosphinothionyl)disulfide (BDPS). This allows to reach the minimum requirement of fire protection (class B2) with only 3% ISTP.[22]
Polymers from Isosorbide
Isosorbide has been examined as a potential platform chemical for the production of diverse polymers and resins.[3] The hydroxy groups can be converted into the primary amino groups[3] via the tosylates and azides or by addition of acrylonitrile followed by hydrogenation into the corresponding aminopropyl derivatives.[23] The latter have potential for the preparation of polyurethanes, as diamines for the preparation of polyamides, and as a hardener for epoxy resins.
When monoethylene glycol as a diol is replaced with isosorbide in the polyester polyethylene terephthalate (PET), polyisosorbide terephthalate (PIT) is obtained, which is characterized by an extreme thermal stability (up to 360 °C under nitrogen). However, the inherently lower reactivity of the secondary hydroxyl groups in isosorbide cause in comparison lower molecular weights and high residual contents of terephthalic acid, which leads to the insufficient chemical stability of the resulting polymers. Therefore, today's polyesters with isosorbide and monoethylene glycol are examined as diol components that show improved properties such as less discoloration.[24][25]
Isosorbide is of also of interest as a precursor to polycarbonates,.[26] It could in principle replace the bisphenol A, which was identified as xenoestrogen. Limitations of isosorbide-based polycarbonates are their unsatisfactory temperature resistance and limited impact resistance, which can be improved though by the addition of comonomers to the isosorbide or by polymer blends.[27]
Isosorbide, a diol, is a precursor to In polyurethanes.[28] or as a building block for the polyol[29] [29] It could be converted to the diisocyanate component[30] as well as a chain extender.[31]
By reacting isosorbide with epichlorohydrin, isosorbide bis-glycidyl ether[32] (a bis-epoxide) is formed, which could be used as a replacement for the analog bisphenol A bis-epoxide. Isosorbide bis-glycidyl ether can be crosslinked to a thermosetting epoxy resins with suitable curing agents, such as polyamines or cyclic acid anhydrides. These resins are used as adhesives, paints or coatings for food cans.[33] Furthermore, polyoxazolidones are described which can be obtained by reaction of isosorbide diglycidyl ethers with diisocyanates.[34] Polyoxazolidones could find use as rigid, highly branched and solvent-resistant thermoset plastics in the electrical and electronics industry.
See also
- Dianhydrohexitols, isomers of isosorbide
References
- ↑ "The Top 300 of 2020". ClinCalc. Retrieved 7 October 2022.
- ↑ "Isosorbide - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.
- 1 2 3 4 Palkovits R, Rose M (2012). "Isosorbide as a Renewable Platform chemical for Versatile Applications – Quo Vadis?". ChemSusChem. 5 (1): 167–176. doi:10.1002/cssc.201100580. PMID 22213713.
- ↑ US 9120806, David James Schreck, Marion McKinley Bradford, Nye Atwood Clinton, Paul Aubry, "Dianhydrosugar production process", issued 2015-09-01, assigned to Iowa Corn Promotion Board
- ↑ US 6670033, Hubbard MA, Wohlers M, Witteler HB, Zey EG, Kvakovszky G, Shockley TH, Charbonneau LF, Kohle N, Rieth J, "Process and products of purification of anhydrosugar alcohols", issued 2003-12-30, assigned to E.I. du Pont de Nemours and Company
- 1 2 US 6867296, Bhatia KK, "Recovery and purification of anhydro sugar alcohols from a vapor stream", issued 2005-03-15, assigned to E.I. du Pont de Nemours and Company
- ↑ Flèche G, Huchette M (1986). "Isosorbide. Preparation, properties and chemistry". Starch (in German). 38 (1): 26–30. doi:10.1002/star.19860380107.
- ↑ Datenblatt. "Isosorbide". Acros. Retrieved 6 January 2013.
- ↑ Datenblatt. "D-Glucose" (PDF). Carl Roth. Retrieved 24 August 2010.
- ↑ Feng X, East A, Hammond W, Ophir Z, Zhang Y, Jaffe M (September 2012). "Thermal analysis characterization of isosorbide-containing thermosets: Isosorbide epoxy as BPA replacement for thermosets industry". Journal of Thermal Analysis and Calorimetry. 109 (3): 1267–1275. doi:10.1007/s10973-012-2581-2. S2CID 94674402.
- ↑ CID 12597 from PubChem
- ↑ Tory DB, ed. (2006). Remington : the science and practice of pharmacy (21st ed.). Philadelphia: Lippincott Williams & Wilkins. p. 1359. ISBN 978-0-7817-4673-1.
- ↑ von Claus JE, Schmidt H, eds. (2007). Pharmakologie und Toxikologie für Studium und Praxis (6th ed.). Stuttgart: Schattauer Verlag. ISBN 978-3-7945-2295-8.
- ↑ WIPO Patent Application WO/2010/115565, Isosorbide monoesters and their use in household applications, invent1: C. Breffa et al., assign1: Cognis IP Management GmbH, veröffentlicht am 14. Oktober 2010
- ↑ Deutksche Offenlegungsschrift DE 10 2007 028 702A1, Verfahren zur Herstellung von Dianhydrohexitol-Diestern, invent1: M. Graß, M. Woelk-Faehrmann, assign1: Evonik Oxeno GmbH, offengelegt am 24. Dezember 2008
- ↑ US 2012220507, M. Grass, H.G. Becker, "2,5-Furan dicarboxylate derivatives, and use thereof as plasticizers", issued 2012-08-30, assigned to Evonik Oxeno GmbH
- ↑ "Phthalate-free plasticizers in PVC" (PDF). Healthy Building Network.
- ↑ "About Dimethyl Isosorbid". Grant Industries. Archived from the original on 7 September 2012.
- ↑ US-Patentanmeldung US 2010/0183913 Lithium cell with iron disulfide cathode and improved electrolyte, invent1: M. Sliger et al., assign1: The Procter & Gamble Co., veröffentlicht am 22. Juli 2010
- ↑ US-Patentanmeldung US 2010/0064574A1 Diesel cycle fuel compositions containing dianhydrohexitols and related products, invent1: R.M. de Almeida, C.R. Klotz Rabello, assign1: Petróleo Brasileiro S.A.-Petrobras, veröffentlicht am 18. März 2010.
- ↑ Europäische Patentanmeldung EP 2,574,615: Verfahren zur Herstellung von Zucker(thio)phosphaten, invent1: Ch. Fleckenstein, H. Denecke, assign1: BASF SE, veröffentlicht am 3. April 2013.
- ↑ J. Wagner: Halogenfreie Flammschutzmittelmischungen für Polystyrol-Schäume (PDF; 15,5 MB), Inauguraldissertation, Universität Heidelberg, Juni 2012.
- ↑ US-Patentanmeldung US 2010/0130759A1 Novel functional compounds with an isosorbide or isosorbide isomer core, production process and uses of these compounds, invent1: J.-P. Gillet, assign1: Arkema Inc., veröffentlicht am 27. Mai 2010
- ↑ US-Patentanmeldung US 2006/0173154A1 Process for making low color poly(ethylene-co-isosorbide) terephthalate polymer, invent1: L. Charbonneau, assign1: E.I. du Pont de Nemours and Company, veröffentlicht am 3. August 2006
- ↑ Bersot JC, Jacquel N, Saint-Loup R, Fuertes P, Rousseau A, Pascault JP, Spitz R, Fenouillot F, Monteil V (2011). "Efficiency Increase of Poly (ethylene terephthalate‐co‐isosorbide terephthalate) Synthesis using Bimetallic Catalytic Systems". Macromolecular Chemistry and Physics. 212 (19): 2114–2120. doi:10.1002/macp.201100146.
- ↑ Hani MA, Chatti S, Kricheldorf HR, Zarrouk H (2007). "Polycondensation of isosorbide and various diols by means of diphosgene characterization by a combination of MALDI and NMR" (PDF). Recent Res. Devel. Organic Chem. 11: 1–11. ISBN 978-81-7895-294-9. Archived from the original (PDF) on 4 March 2016. Retrieved 15 March 2018.
- ↑ US-Patentanmeldung US 2011/0160422A1 Isosorbide-based polycarbonates, method for making, and articles formed therefrom, invent1: J.H. Kamps et al., assign1: Sabic Innovative Plastics IP BV, veröffentlicht am 30. Juni 2011
- ↑ PCT-Patent WO 2012/163845 Fibre composite component and a process for the production thereof, invent1: S. Lindner et al., assign1: Bayer IP GmbH, veröffentlicht am 6. Dezember 2012
- ↑ Feng X, East AJ, Hammond W, Jaffe M (2010). "Sugar-based chemicals for environmentally sustainable applications.". Contemporary Science of Polymeric Materials: A Symposium in honor of Professor Frank E. Karasz on the occasion of his 75th birthday. American Chemical Society. pp. 3–27. ISBN 978-0-8412-2602-9.
- ↑ Bachmann F, Reimer J, Ruppenstein M, Thiem J (2001). "Synthesis of Novel Polyurethanes and Polyureas by Polyaddition Reactions of Dianhydrohexitol Configurated Diisocyanates". Macromolecular Chemistry and Physics (in German). 202 (17): 3410–3419. doi:10.1002/1521-3935(20011101)202:17<3410::AID-MACP3410>3.0.CO;2-Q0 (inactive 1 August 2023).
{{cite journal}}: CS1 maint: DOI inactive as of August 2023 (link) - ↑ US-Patentanmeldung US 2011/0015366 A1 Novel chain extenders for polyurethane elastomer formulations, invent1: G. da Costa et al., veröffentlicht am 20. Januar 2011
- ↑ US-Patent US 7,619,056B2 Thermoset epoxy polymers from renewable resources, invent1: A.J. East et al., assign1: New Jersey Institute of Technology, erteilt am 17. November 2009
- ↑ Press Release NEWARK, 24 February 2010: NJIT Patent May Be Able To Replace BPA; Make Consumer Products Safer
- ↑ US-Patentanmeldung US 2010/0298520A1 Polyoxazolidones derived from dianhydrohexitols, invent1: A.J. East et al., assign1: New Jersey Institute of Technology, veröffentlicht am 25. November 2010