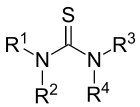
In organic chemistry, thioureas are members of a family of organosulfur compounds with the formula S=C(NR2)2 and structure R2N−C(=S)−NR2. The parent member of this class of compounds is thiourea (S=C(NH2)2). Substituted thioureas are found in several commercial chemicals.
Structure and bonding
Thioureas have planar N2C=S core. The C=S bond distance is near 1.71 Å, which is 0.1 Å longer than in normal ketones (R2C=O). The C–N bond distances are short.[1] Thioureas occurs in two tautomeric forms. For the parent thiourea, the thione form predominates in aqueous solutions.[2] The thiol form, known as an isothiourea, can be encountered in substituted compounds such as isothiouronium salts.
On the other hand, some compounds depicted as isothioureas and in fact thioureas, one example being mercaptobenzimidazole.[3]
Synthesis
N,N′-unsubstituted thioureas can be prepared by treating the corresponding cyanamide with hydrogen sulfide or similar sulfide sources.[4] Organic ammonium salts react with potassium thiocyanate as the source of the thiocarbonyl (C=S).[5]
Alternatively, N,N′-disubstituted thioureas can be prepared by coupling two amines with thiophosgene:[6]
Amines also condense with organic thiocyanates to give thioureas:[7]
Cyclic thioureas are prepared by transamidation of thiourea with diamines. Ethylene thiourea is synthesized by treating ethylenediamine with carbon disulfide.[8] In some cases, thioureas can be prepared by thiation of ureas using phosphorus pentasulfide.

Applications
Agrichemicals that feature the thiourea functional group include methimazole, carbimazole (converted in vivo to methimazole), and propylthiouracil.
Catalysis
Some thioureas are vulcanization accelerators. Thioureas are also used in a research theme called thiourea organocatalysis.[9]
References
- ↑ D. Mullen; E. Hellner (1978). "A Simple Refinement of Density Distributions of Bonding Electrons. IX. Bond Electron Density Distribution in Thiourea, C=S(NH2)2, at 123K". Acta Crystallogr. B34 (9): 2789–2794. doi:10.1107/S0567740878009243.
- ↑ Allegretti, P.E; Castro, E.A; Furlong, J.J.P (March 2000). "Tautomeric equilibrium of amides and related compounds: theoretical and spectral evidences". Journal of Molecular Structure: THEOCHEM. 499 (1–3): 121–126. doi:10.1016/S0166-1280(99)00294-8.
- ↑ Form, G. R.; Raper, E. S.; Downie, T. C. (1976). "The crystal and molecular structure of 2-mercaptobenzimidazole". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry. 32 (2): 345–348. doi:10.1107/S0567740876003026.
- ↑ Koketsu, Mamoru; Kobayashi, Chikashi; Ishihara, Hideharu (2003). "Synthesis of N-aryl-S-alkylthiocarbamates". Heteroatom Chemistry. 14 (4): 374–378. doi:10.1002/hc.10163.
- ↑ Herr, R. J.; Kuhler, L.; Meckler, H.; Opalka, C. J. (2000). "A Convenient Method for the Preparation of Primary and Symmetrical N,N′-Disubstituted Thioureas". Synthesis. 2000 (11): 1569–1574. doi:10.1055/s-2000-7607.
- ↑ Yi-Bo Huang; Wen-Bin Yi; Chun Cai (2012). "Thiourea Based Fluorous Organocatalyst". Topics in Current Chemistry. 308: 191–212. doi:10.1007/128_2011_248. ISBN 978-3-642-25233-4. PMID 21972024.
- ↑ Miyabe, H.; Takemoto, Y. (2008). "Discovery and Application of Asymmetric Reaction by Multifunctional Thioureas". Bull Chem Soc Jpn. 81 (7): 785. doi:10.1246/bcsj.81.785.
- ↑ C. F. H. Allen; C. O. Edens; James VanAllan. "Ethylene Thiourea". Org. Syntheses. 26: 34. doi:10.15227/orgsyn.026.0034.
- ↑ R. Schreiner, Peter (2003). "Metal-free organocatalysis through explicit hydrogen bonding interactions". Chem. Soc. Rev. 32 (5): 289–296. doi:10.1039/b107298f. PMID 14518182.
Further reading
- Patai, S., ed. (1977). The Chemistry of double-bonded functional groups. New York, NY: John Wiley & Sons. pp. 1355–1496. ISBN 0-471-92493-8.
.png.webp)