In chemistry, an electron pair or Lewis pair consists of two electrons that occupy the same molecular orbital but have opposite spins. Gilbert N. Lewis introduced the concepts of both the electron pair and the covalent bond in a landmark paper he published in 1916.[1][2]
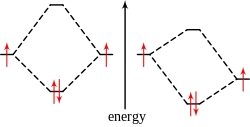
Because electrons are fermions, the Pauli exclusion principle forbids these particles from having the same quantum numbers. Therefore, for two electrons to occupy the same orbital, and thereby have the same orbital quantum number, they must have different spin quantum number. This also limits the number of electrons in the same orbital to two.
The pairing of spins is often energetically favorable, and electron pairs therefore play a large role in chemistry. They can form a chemical bond between two atoms, or they can occur as a lone pair of valence electrons. They also fill the core levels of an atom.
Because the spins are paired, the magnetic moment of the electrons cancel one another, and the pair's contribution to magnetic properties is generally diamagnetic.
Although a strong tendency to pair off electrons can be observed in chemistry, it is also possible that electrons occur as unpaired electrons.
In the case of metallic bonding the magnetic moments also compensate to a large extent, but the bonding is more communal so that individual pairs of electrons cannot be distinguished and it is better to consider the electrons as a collective 'sea'.
A very special case of electron pair formation occurs in superconductivity: the formation of Cooper pairs. In unconventional superconductors, whose crystal structure contains copper anions, the electron pair bond is due to antiferromagnetic spin fluctuations.[3]
See also
References
- ↑ Lewis, G. N. (1916). The atom and the molecule. Journal of the American Chemical Society, 38(4), 762-785.
- ↑ Jean Maruani (1989). Molecules in Physics, Chemistry and Biology: v. 3: Electronic Structure and Chemical Reactivity. Springer. p. 73. ISBN 978-90-277-2598-1. Retrieved 14 March 2013.
- ↑ Vienna University of Technology (August 16, 2022). "Study identifies mechanism holding electron pairs together in unconventional superconductors". Phys.org.
To stay with the solar system analogy, the spin fluctuations thus correspond to that reference system in which the sun is placed at the center.