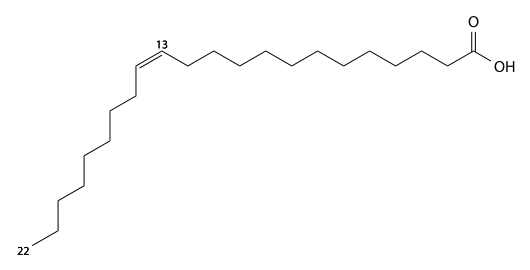
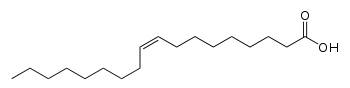
Lorenzo's oil is a liquid solution made of 4 parts glycerol trioleate and 1 part glycerol trierucate, which are the triacylglycerol forms of oleic acid and erucic acid.[1] It is prepared from olive oil and rapeseed oil.[2] It is used in the investigational treatment of asymptomatic patients with adrenoleukodystrophy (ALD), a nervous system disorder.
The development of the oil was led by Augusto and Michaela Odone after their son Lorenzo was diagnosed with the disease in 1984, at the age of five. Lorenzo was predicted to die within a few years. His parents sought experimental treatment options, and the initial formulation of the oil was developed by retired British scientist Don Suddaby (formerly of Croda International).[3] Suddaby and his colleague, Keith Coupland, received a U.S. patent (since expired) for invention of the oil.[4] The royalties received by Augusto were paid to The Myelin Project which he and Michaela founded to further research treatments for ALD and similar disorders. The Odones and their invention obtained widespread publicity in 1992 because of the film Lorenzo's Oil.
Research on the effectiveness of Lorenzo's oil has seen mixed results, with possible benefit for asymptomatic ALD patients but of unpredictable or no benefit to those with symptoms, suggesting its possible role as a preventative measure in families identified as ALD dominant. Lorenzo Odone died on May 30, 2008, at the age of 30; he was bedridden with paralysis and died from aspiration pneumonia, likely caused by having inhaled food.[5][6]
Treatment cost
In 2012, Lorenzo's oil cost approximately $400 USD for a month's treatment.[7]
Proposed mechanism of action
The mixture of fatty acids purportedly reduces the levels of very long chain fatty acids (VLCFAs), which are elevated in ALD. It does so by competitively inhibiting the enzyme that forms VLCFAs.[8]
Effectiveness
Lorenzo's oil, in combination with a diet low in VLCFA, has been investigated for its possible effects on the progression of ALD. Clinical results have been mixed and the use of Lorenzo's oil has been controversial due to uncertainties regarding its clinical efficacy and the clinical indications for its use.[9]
Hugo Moser played a prominent role in both the treatment of Lorenzo Odone and the scientific evaluation of Lorenzo's oil. In 2005, Moser published a controlled study concluding that Lorenzo's oil does not alter the course of the illness in symptomatic patients, but asymptomatic patients had a reduced risk of developing ALD while on the dietary therapy.[10] Moser appraised Lorenzo's oil again in a 2007 report.[11]
Moser's findings, that Lorenzo's oil did not help symptomatic ALD patients, are consistent with prior studies published in 2003[12] and 1999.[9][13]
A study by Poulos published in 1994 found that Lorenzo's oil is of limited value in correcting the accumulation of saturated VLCFAs in the brain of patients with ALD.[14] Comparative autopsies showed that treatment enriched erucic acid in plasma and tissues, but not in the brain.[15]
Side effects
The oil has been shown to cause a lowered platelet count,[16] which can lead to thrombocytopenia and lymphopenia.[17]: 646–657
There are no reports of toxicity from dietary consumption of erucic acid.[17][18][19]
Current state
Dietary manipulation using Lorenzo's oil has been shown to lower blood levels of very long chain fatty acids, but it is ineffective in symptomatic ALD. Moser's 2005 study has found "strongly suggestive, albeit not fully definitive, evidence of a preventive effect" of Lorenzo's oil on the onset of symptoms when used by asymptomatic patients.[10]
References
- ↑ "Page Not Found". www.lowproteinconnect.com. 20 October 2020. Archived from the original on 27 June 2020. Retrieved 13 July 2022.
{{cite web}}: Cite uses generic title (help) - ↑ Shankar Vedantam. "A Real-Life Sequel to 'Lorenzo's Oil' – washingtonpost.com". Washington Post 2007-01-28. pp. A01. Retrieved 2007-12-10.
- ↑ "Lorenzo Odone". 1 June 2008 – via www.telegraph.co.uk.
- ↑ U.S. Patent 5,331,009: Pharmaceutical compositions for treating adrenoleukodystrophy. Issued July 19, 1994.
- ↑ "Lorenzo's Oil boy is dead at 30". BBC News. 31 May 2008. Retrieved 2008-06-02.
- ↑ "Subject of 'Lorenzo's Oil' dies at 30". CNN. Associated Press. 2008-05-30. Archived from the original on July 17, 2008. Retrieved 2008-07-12.
- ↑ "Lorenzo's Oil – The Oil". The Myelin Project. Retrieved 3 November 2012.
- ↑ "Archived copy". www.myelin.org. Archived from the original on 4 November 2013. Retrieved 17 January 2022.
{{cite web}}: CS1 maint: archived copy as title (link) - 1 2 Berger J, Pujol A, Aubourg P, Forss-Petter S (July 2010). "Current and Future Pharmacological Treatment Strategies in X-Linked Adrenoleukodystrophy". Brain Pathol. 20 (4): 845–56. doi:10.1111/j.1750-3639.2010.00393.x. PMC 2967711. PMID 20626746.
- 1 2 Moser HW, Raymond GV, Lu SE, Muenz LR, Moser AB, Xu J, Jones RO, Loes DJ, Melhem ER, Dubey P, Bezman L, Brereton NH, Odone A (July 2005). "Follow-up of 89 asymptomatic patients with adrenoleukodystrophy treated with Lorenzo's Oil". Archives of Neurology. 62 (7): 1073–80. doi:10.1001/archneur.62.7.1073. PMID 16009761.
- ↑ Moser HW, Moser AB, Hollandsworth K, Brereton NH, Raymond GV (September 2007). ""Lorenzo's oil" therapy for X-linked adrenoleukodystrophy: rationale and current assessment of efficacy". J. Mol. Neurosci. 33 (1): 105–13. doi:10.1007/s12031-007-0041-4. PMID 17901554. S2CID 21333247.
- ↑ Aubourg P, Adamsbaum C, Lavallard-Rousseau MC, Rocchiccioli F, Cartier N, Jambaqué I, Jakobezak C, Lemaitre A, Boureau F, Wolf C (September 1993). "A two-year trial of oleic and erucic acids ("Lorenzo's oil") as treatment for adrenomyeloneuropathy". N. Engl. J. Med. 329 (11): 745–52. doi:10.1056/NEJM199309093291101. PMID 8350883.
- ↑ van Geel BM, Assies J, Haverkort EB, Koelman JH, Verbeeten B, Wanders RJ, Barth PG (September 1999). "Progression of abnormalities in adrenomyeloneuropathy and neurologically asymptomatic X-linked adrenoleukodystrophy despite treatment with "Lorenzo's oil"". J. Neurol. Neurosurg. Psychiatry. 67 (3): 290–9. doi:10.1136/jnnp.67.3.290. PMC 1736534. PMID 10449548.
- ↑ Poulos A, Gibson R, Sharp P, Beckman K, Grattan-Smith P (1994). "Very long chain fatty acids in X-linked adrenoleukodystrophy brain after treatment with Lorenzo's oil". Ann. Neurol. 36 (5): 741–6. doi:10.1002/ana.410360509. PMID 7979219. S2CID 41340913.
- ↑ Magnhild Rasmussen; Ann B. Moser; Janet Borel; Surinder Khangoora; Hugo W. Moser (Aug 1994). "Brain, liver, and adipose tissue erucic and very long chain fatty acid levels in adrenoleukodystrophy patients treated with glyceryl trierucate and trioleate oils (Lorenzo's Oil)". Neurochemical Research. Springer Netherlands. 19 (8): 1073–1082. doi:10.1007/BF00968719. PMID 7800117. S2CID 11658824.
- ↑ Crowther MA, Barr RD, Kelton J, Whelan D, Greenwald M (February 1995). "Profound thrombocytopenia complicating dietary erucic acid therapy for adrenoleukodystrophy". American Journal of Hematology. 48 (2): 132–3. doi:10.1002/ajh.2830480217. PMID 7847331. S2CID 29556389.
- 1 2 Luger CL et al. Food Safety and Foodborne Toxicants. Chapter 14 in Hayes' Principles and Methods of Toxicology, Sixth Edition. Eds A. Wallace Hayes, Claire L. Kruger. CRC Press, 2014 ISBN 9781842145371. Quote: "In humans. however. although the long-term use of Lorenzo's oil (oleic acid and erucic acid) in the treatment of adrenoleukodystrophy or adrenomyeloneuropathy leads to thrombocytopenia and lymphopenia (Unkrig et al. 1994), adverse effects from dietary consumption of erucic acid have not been reported."
- ↑ Food Standards Australia New Zealand (June 2003) Erucic acid in food Archived 2008-12-03 at the Wayback Machine : A Toxicological Review and Risk Assessment . Technical report series No. 21; Page 4 paragraph 1; ISBN 0-642-34526-0, ISSN 1448-3017
- ↑ "Food Standards Agency - Agency issues warning on erucic acid". 2 September 2004. Retrieved 2007-11-02.
External links
- The Myelin Project (international site)
- Lorenzo and His Parents (official site of book)