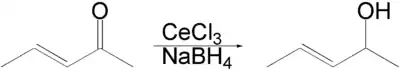| Luche reduction | |
|---|---|
| Named after | Jean-Louis Luche |
| Reaction type | Organic redox reaction |
| Identifiers | |
| Organic Chemistry Portal | luche-reduction |
| RSC ontology ID | RXNO:0000286 |
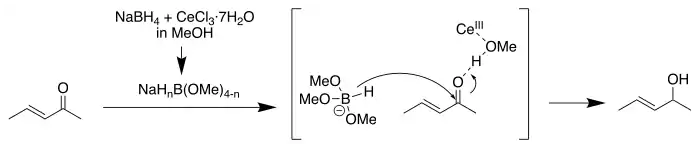
Luche reduction is the selective organic reduction of α,β-unsaturated ketones to allylic alcohols.[1][2][3] The active reductant is described as "cerium borohydride", which is generated in situ from NaBH4 and CeCl3(H2O)7.[4]
The Luche reduction can be conducted chemoselectively toward ketone in the presence of aldehydes or towards α,β-unsaturated ketones in the presence of a non-conjugated ketone.[5]
An enone forms an allylic alcohol in a 1,2-addition, and the competing conjugate 1,4-addition is suppressed.
The selectivity can be explained in terms of the HSAB theory: carbonyl groups require hard nucleophiles for 1,2-addition. The hardness of the borohydride is increased by replacing hydride groups with alkoxide groups, a reaction catalyzed by the cerium salt by increasing the electrophilicity of the carbonyl group. This is selective for ketones because they are more Lewis basic.
In one application, a ketone is selectively reduced in the presence of an aldehyde. Actually, in the presence of methanol as solvent, the aldehyde forms a methoxy acetal that is inactive in the reducing conditions.
References
- ↑ Kürti, László; Czakó, Barbara (2005). Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms. Elsevier Academic Press. ISBN 0-12-429785-4.
- ↑ Luche, J.-L. (1978). "Lanthanides in Organic Chemistry. 1. Selective 1,2 Reductions of Conjugated Ketones". J. Am. Chem. Soc. 100 (7): 2226–2227. doi:10.1021/ja00475a040.
- ↑ Luche, J.-L.; Rodriguez-Hahn, L.; Crabbé, P. (1978). "Reduction of Natural Enones in the Presence of Cerium Trichloride". J. Chem. Soc., Chem. Commun. (14): 601–602. doi:10.1039/C39780000601.
- ↑ Paquette, Leo A.; Sabitha, G.; Yadav, J. S.; Scheuermann, Angelique M.; Merchant, Rohan R. (2021). "Cerium(III) Chloride". Encyclopedia of Reagents for Organic Synthesis. pp. 1–15. doi:10.1002/047084289X.rc041.pub3. ISBN 9780471936237.
- ↑ Gemal, A. L.; Luche, J.-L. (1981). "Lanthanoids in Organic Synthesis. 6. The Reduction of α-Enones by Sodium Borohydride in the Presence of Lanthanoid Chlorides: Synthetic and Mechanistic Aspects". J. Am. Chem. Soc. 103 (18): 5454–5459. doi:10.1021/ja00408a029.