| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,3-Xylene[1] | |||
| Systematic IUPAC name
1,3-Dimethylbenzene | |||
| Other names
m-Xylene,[1] meta-Xylol | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 605441 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.252 | ||
| EC Number |
| ||
| 101390 | |||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1307 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C8H10 | |||
| Molar mass | 106.16 g/mol | ||
| Appearance | Colorless liquid | ||
| Odor | Aromatic in high concentrations | ||
| Density | 0.86 g/mL | ||
| Melting point | −48 °C (−54 °F; 225 K) | ||
| Boiling point | 139 °C (282 °F; 412 K) | ||
| does not react with water | |||
| Solubility in ethanol | miscible | ||
| Solubility in diethyl ether | miscible | ||
| Vapor pressure | 9 mmHg (20°C)[2] | ||
| -76.56·10−6 cm3/mol | |||
Refractive index (nD) |
1.49722 | ||
| Viscosity | 0.8059 cP at 0 °C 0.6200 cP at 20 °C | ||
| 0.33-0.37 D[3] | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
Harmful if swallowed. Vapor harmful. Flammable liquid and vapor. | ||
| GHS labelling: | |||
   | |||
| Danger | |||
| H226, H302, H304, H312, H315, H318, H332 | |||
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P301+P310, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P322, P331, P332+P313, P362, P363, P370+P378, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 27 °C (81 °F; 300 K)[4] | ||
| 527 °C (981 °F; 800 K)[4] | |||
| Explosive limits | 1.1%-7.0%[2] | ||
Threshold limit value (TLV) |
100 ppm[4] (TWA), 150 ppm[4] (STEL) | ||
| Lethal dose or concentration (LD, LC): | |||
LCLo (lowest published) |
2010 ppm (mouse, 24 hr) 8000 ppm (rat, 4 hr)[5] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 100 ppm (435 mg/m3)[2] | ||
REL (Recommended) |
TWA 100 ppm (435 mg/m3) ST 150 ppm (655 mg/m3)[2] | ||
IDLH (Immediate danger) |
900 ppm[2] | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Related aromatic hydrocarbons |
benzene toluene o-xylene p-xylene | ||
| Supplementary data page | |||
| M-Xylene (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
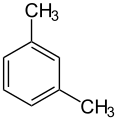
m-Xylene (meta-xylene) is an aromatic hydrocarbon. It is one of the three isomers of dimethylbenzene known collectively as xylenes. The m- stands for meta-, indicating that the two methyl groups in m-xylene occupy positions 1 and 3 on a benzene ring. It is in the positions of the two methyl groups, their arene substitution pattern, that it differs from the other isomers, o-xylene and p-xylene. All have the same chemical formula C6H4(CH3)2. All xylene isomers are colorless and highly flammable.[6]
Production and use
Petroleum contains about 1 weight percent xylenes.[7] The meta isomer can be isolated from a mix of xylenes by the partial sulfonation (to which other isomers are less prone) followed by removal of unsulfonated oils and steam distillation of the sulfonated product.
The major use of meta-xylene is in the production of isophthalic acid, which is used as a copolymerizing monomer to alter the properties of polyethylene terephthalate. The conversion m-xylene to isophthalic acid entails catalytic oxidation. meta-Xylene is also used as a raw material in the manufacture of 2,4- and 2,6-xylidine as well as a range of smaller-volume chemicals.[8][6] Ammoxidation gives isophthalonitrile.
Toxicity and exposure
Xylenes are not acutely toxic, for example the LD50 (rat, oral) is 4300 mg/kg. Effects vary with animal and xylene isomer. Concerns with xylenes focus on narcotic effects.[6]
See also
References
- 1 2 Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 139. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- 1 2 3 4 5 NIOSH Pocket Guide to Chemical Hazards. "#0669". National Institute for Occupational Safety and Health (NIOSH).
- ↑ DeanHandb, Lange´s Handbook of chemistry, 15th edition,1999.
- 1 2 3 4 "m-Xylene". International Chemical Safety Cards. IPCS/NIOSH. July 1, 2014. Archived from the original on December 5, 2017. Retrieved September 9, 2017.
- ↑ "Xylene (o-, m-, p-isomers)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 Fabri, Jörg; Graeser, Ulrich; Simo, Thomas A. (2000). "Xylenes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a28_433. ISBN 978-3527306732.
- ↑ EPA-454/R-93-048 Locating and estimating air emissions from sources of xylene Emission Inventory Branch Technical Support Division Office of Air Quality Planning and Standards U.S. Environmental Protection Agency March 1994
- ↑ Ashford's Dictionary of Industrial Chemicals, third edition, page 9692.