| Eumycetoma | |
|---|---|
| Other names | Madura foot[1] |
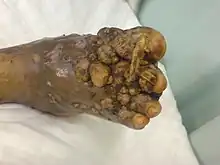 | |
| An infected foot | |
| Specialty | Infectious disease[2] |
| Symptoms | Swelling, weeping pus filled sinuses, deformity.[3] |
| Complications | Amputation |
| Causes | Madurella spp., Falciformispora senegalensis, Curvularia lunata, Pseudallescheria spp., Neotestudina spp., Acremonium spp., Scedosporium spp. and Fusarium spp. [2] |
| Diagnostic method | Microscopy, biopsy, culture,[3] medical imaging, ELISA, immunodiffusion, DNA sequencing[4] |
| Differential diagnosis | Actinomycosis (Actinomycetoma)[3] |
| Treatment | Surgical debridement, antifungal medicines[3] |
| Medication | Itraconazole, posaconazole, voriconazole[4] |
| Prognosis | Recurrence is common[5] |
| Frequency | Endemic in Africa, India and South America[3] |
Eumycetoma, also known as Madura foot,[1][6] is a persistent fungal infection of the skin and the tissues just under the skin, affecting most commonly the feet, although it can occur in hands and other body parts.[5] It starts as a painless wet nodule, which may be present for years before ulceration, swelling, grainy discharge and weeping from sinuses and fistulae, followed by bone deformity.[3]
Several fungi can cause eumycetoma,[5] including: Madurella mycetomatis, Madurella grisea, Curvularia lunata, Scedosporium species, Acremonium and Fusarium species.[2] Diagnosis is by biopsy, visualising the fungi under the microscope and culture.[5] Medical imaging may reveal extent of bone involvement.[4] Other tests include ELISA, immunodiffusion, and DNA Barcoding.[4]
Treatment includes surgical removal of affected tissue and antifungal medicines.[3] After treatment, recurrence is common.[5] Sometimes amputation is required.[5]
The infection occurs generally in the tropics,[7] and is endemic in Sub-Saharan Africa, especially Sudan, India, parts of South America and Mexico.[3] Few cases have been reported across North Africa.[8][9] Mycetoma is probably low-endemic to Egypt with predilection for eumycetoma.[10] In 2016, the World Health Organization recognised eumycetoma as a neglected tropical disease.[7]
Signs and symptoms

The initial lesion is a small swelling under the skin following minor trauma which breaches the skin.[11][12] It appears as a painless wet nodule, which may be present for years before ulceration, swelling and weeping from sinuses, followed by bone deformity.[3][7] The sinuses discharge a grainy liquid of fungal colonies.[11] These grains are usually black or white.[13] Destruction of deeper tissues, and deformity and loss of function in the affected limbs may occur in later stages.[14] It tends to occur in one foot.[13] Mycetoma due to bacteria has similar clinical features.[15]
Causes
Eumycetoma is a type of mycetoma caused by fungi, distinct from mycetoma caused by bacteria from the phylum Actinomycetes;[11][12] both have similar clinical features.[15]
The most common fungi causing white discharge is Scedosporium (ex. Pseudoalleschia) boydii.[13][16] Other causative agents of non-black grain eumycetoma include Acremonium and Fusarium species.[13]
Black discharge tends to be caused by species from the genera Madurella, Pyrenochaeta, Exophiala, Leptosphaeria and Curvularia.[13] The most common species are Madurella mycetomatis[13][17] and Trematospheria grisea (previously called Madurella grisea).[13][18]
Other fungal causative agents include:
- Acremonium falciform[5][19]
- Acremonium kiliense[5][19]
- Acremonium recifei[5][19]
- Aspergillus flavus[5][19]
- Aspergillus nidulans[5][19]
- Cladophialophora bantiana[5][19]
- Cladophialophora mycetomatis[5][19]
- Curvularia geniculata[5][19]
- Curvularia lunata[5][19][2][20]
- Cylindrocarpon cyanescens[5][19]
- Exophiala jeanselmei[5][19]
- Falciformispora senegalensis[20]
- Fusarium moniliforme[5][19]
- Fusarium solani[5][19]
- Glenospora clapieri[19]
- Leptosphaeria senegalensis[5][19][2]
- Leptosphaeria tompkinsii[5][19]
- Madurella grisea[5][19][2]
- Madurella mycetomatis[5][19][2]
- Microsporum audouinii[5][19]
- Microsporum canis[5][19]
- Neotestudina rosatii[5][19][2]
- Phaeoacremonium parasiticum[19]
- Phialophora cyanescens[19]
- Phialophora verrucosa[5][19]
- Scedosporium (ex. Pseudoalleschia) boydii[5][19][2]
- Pyrenochaeta mackinonii[5][19]
- Pyrenochaeta romeroi[5][19]
- Trichophyton rubrum[5][19]
- Zopfia rosatii[20]
Mechanism
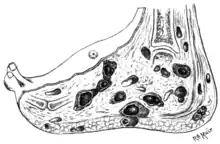
The disease is acquired by entry of the fungal spores from the soil through a breach in the skin produced by minor trauma like a thorn prick.[21] The disease then spreads to deeper tissues and also forms sinus tracts leading to skin surface.[12] Mature lesions are characterised by a grainy discharge from these sinuses. These discharges contain fungal colonies and are infective. Spread of infection internally through blood or lymph is uncommon.
Infections that produce a black discharge mainly spread subcutaneously. In the red and yellow varieties deep spread occurs early, infiltrating muscles and bones but sparing nerves and tendons, which are highly resistant to the invasion.[22]
Botryomycosis, also known as bacterial pseudomycosis, produces a similar clinical picture and is caused usually by Staphylococcus aureus.[23] Other bacteria may also cause botryomycosis.[24]
Diagnosis
Diagnosis is by biopsy, visualising the fungi under the microscope and culture, which show characteristic fungal filaments and vesicles characteristic of the fungi.[5] Other tests include ELISA, immunodiffusion, and PCR with DNA sequencing (so-called DNA barcoding).[4]
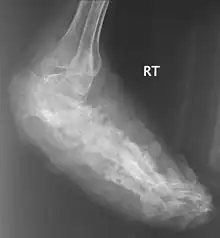
X rays and ultrasonography may be carried out to assess the extent of the disease. X rays findings are extremely variable. The disease is most often observed at an advanced stage that exhibits extensive destruction of all bones of the foot. Rarely, a single lesion may be seen in the tibia where the picture is identical with chronic osteomyelitis. Cytology of fine needle aspirate or pus from the lesion, and tissue biopsy may be undertaken sometimes.[11] Some publications have claimed a "dot in a circle sign" as a characteristic MRI feature for this condition (this feature has also been described on ultrasound).[14]
Differential diagnosis
The following clinical conditions may be considered before diagnosing a patient with mycetoma:
- Tuberculous ulcer
- Kaposi's sarcoma, a vascular tumour of skin usually seen in AIDS.
- Leprosy
- Syphilis
- Malignant neoplasm
- Tropical ulcer[22]
- Botryomycosis,[12] a skin infection usually caused by the bacteria Staphylococcus aureus.
Prevention
No vaccine is available. Simple hygienic precautions like wearing shoes or sandals while working in fields, and washing hands and feet at regular intervals may help prevent the disease.
Treatment
Surgery combined with itraconazole may be given for up to year when the grains are black.[4] Posaconazole is another option.[4] Voriconazole can be used for infections caused by Fusarium species.[4]
Ketoconazole has been used to treat eumycetoma but has many side effects.[25] Actinomycetes usually respond well to medical treatment, but eukaryotic infections are generally resistant and may require surgical interventions including salvage procedures as bone resection or even the more radical amputation.[26][12][14]
Oral fosravuconazole, which is much cheaper than itraconazole, an important factor as eumycetoma mainly affects young adults in poorer, rural areas, was found in 2023 in Phase II clinical trials to be safe, patient-friendly, and effective in treating eumycetoma.[27][28]
Epidemiology
The disease is more common in males aged 20–40 years who work as labourers, farmers and herders, and in travellers to tropical regions, where the condition is endemic.[4]
History
Madura foot or maduromycosis or maduramycosis[29] is described in ancient writings of India as Padavalmika, which, translated means Foot anthill.[12] The first modern description of Madura foot was made in 1842 from Madurai (the city after which the disease was named Madura-mycosis) in India, by Gill.[12] The fungal cause of the disease was established in 1860 by Carter.[12]
Society and culture
In 2016, the World Health Organization recognised eumycetoma as a neglected tropical disease.[7] Traditionally occurring in regions where resources are scarce, medicines may be expensive and diagnosis is frequently made late, when more invasive treatment may be required.[7]
References
- 1 2 Kutzner H, Kempf W, Feit J, Sangueza O (2021). "2. Fungal infections". Atlas of Clinical Dermatopathology: Infectious and Parasitic Dermatoses. Hoboken: Wiley Blackwell. pp. 77–108. ISBN 978-1-119-64706-5. Archived from the original on 10 June 2021. Retrieved 9 June 2021.
- 1 2 3 4 5 6 7 8 9 "ICD-11 – ICD-11 for Mortality and Morbidity Statistics". icd.who.int. Archived from the original on 1 August 2018. Retrieved 9 June 2021.
- 1 2 3 4 5 6 7 8 9 Johnstone RB (2017). "25. Mycoses and Algal infections". Weedon's Skin Pathology Essentials (2nd ed.). Elsevier. p. 457. ISBN 978-0-7020-6830-0. Archived from the original on 25 May 2021. Retrieved 13 June 2021.
- 1 2 3 4 5 6 7 8 9 Queiroz-Telles F, Fahal AH, Falci DR, Caceres DH, Chiller T, Pasqualotto AC (November 2017). "Neglected endemic mycoses". The Lancet. Infectious Diseases. 17 (11): e367–e377. doi:10.1016/S1473-3099(17)30306-7. ISSN 1474-4457. PMID 28774696. Archived from the original on 27 August 2021. Retrieved 30 August 2021.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 Estrada R, Chávez-López G, Estrada-Chávez G, López-Martínez R, Welsh O (July 2012). "Eumycetoma". Clinics in Dermatology. 30 (4): 389–396. doi:10.1016/j.clindermatol.2011.09.009. ISSN 1879-1131. PMID 22682186.
- ↑ Barlow G, Irving I, moss PJ (2020). "20. Infectious diseases". In Feather A, Randall D, Waterhouse M (eds.). Kumar and Clark's Clinical Medicine (10th ed.). Elsevier. p. 561. ISBN 978-0-7020-7870-5. Archived from the original on 13 June 2021. Retrieved 13 June 2021.
- 1 2 3 4 5 Emery D, Denning DW (2020). "The global distribution of actinomycetoma and eumycetoma". PLOS Neglected Tropical Diseases. 14 (9): e0008397. doi:10.1371/journal.pntd.0008397. ISSN 1935-2735. PMC 7514014. PMID 32970667.
- ↑ Elgallali N, El Euch D, Cheikhrouhou R, Belhadj S, Chelly I, Chaker E, Ben Osman A (June 2010). "[Mycetoma in Tunisia: a 15-case series]". Médecine Tropicale. 70 (3): 269–73. PMID 20734597.
- ↑ Karrakchou B, Boubnane I, Senouci K, Hassam B (10 January 2020). "Madurella mycetomatis infection of the foot: a case report of a neglected tropical disease in a non-endemic region". BMC Dermatology. 20 (1): 1. doi:10.1186/s12895-019-0097-1. PMC 6953183. PMID 31918687.
- ↑ Ahmed SA, El-Sobky TA, de Hoog S, Zaki SM, Taha M (9 September 2022). "A scoping review of mycetoma profile in Egypt: revisiting the global endemicity map". Transactions of the Royal Society of Tropical Medicine and Hygiene. 117 (1): 1–11. doi:10.1093/trstmh/trac085. PMC 9808524. PMID 36084235.
- 1 2 3 4 Davidson's principles and practice of medicine (20th ed.). Churchill Livingstone Elsevier. 2006. p. 373. ISBN 9780443101335.
- 1 2 3 4 5 6 7 8 Ananthanarayan BA, Jayaram CK, Paniker MD (2006). Textbook of Microbiology (7th ed.). Orient Longman Private Ltd. p. 618. ISBN 978-8125028086.
- 1 2 3 4 5 6 7 Bravo FG (2020). "14. Fungal, viral and rickettsial infections". In Hoang MP, Selim MA (eds.). Hospital-Based Dermatopathology: An Illustrated Diagnostic Guide. Switzerland: Springer. pp. 638–664. ISBN 978-3-030-35819-8. Archived from the original on 11 June 2021. Retrieved 11 June 2021.
- 1 2 3 El-Sobky TA, Haleem JF, Samir S (2015). "Eumycetoma Osteomyelitis of the Calcaneus in a Child: A Radiologic-Pathologic Correlation following Total Calcanectomy". Case Reports in Pathology. 2015: 129020. doi:10.1155/2015/129020. PMC 4592886. PMID 26483983.
- 1 2 "Mycetoma | DermNet NZ". dermnetnz.org. Archived from the original on 13 June 2021. Retrieved 11 June 2021.
- ↑ "Filamentous Fungi". Archived from the original on 18 June 2012. Retrieved 23 July 2008.
- ↑ Ahmed AO, Desplaces N, Leonard P, et al. (December 2003). "Molecular detection and identification of agents of eumycetoma: detailed report of two cases". J. Clin. Microbiol. 41 (12): 5813–6. doi:10.1128/JCM.41.12.5813-5816.2003. PMC 309011. PMID 14662990.
- ↑ Vilela R, Duarte OM, Rosa CA, et al. (November 2004). "A case of eumycetoma due to Madurella grisea in northern Brazil" (PDF). Mycopathologia. 158 (4): 415–8. doi:10.1007/s11046-004-2844-y. PMID 15630550. S2CID 35337823.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 J. Alexandro Bonifaz Trujillo, Micología médica básica. México: McGraw Hill; 2010. ISBN 978-607-15-0744-0
- 1 2 3 WHO fungal priority pathogens list to guide research, development and public health action (PDF). 25 October 2022. ISBN 978-92-4-006024-1. Archived from the original on 26 October 2022. Retrieved 27 October 2022.
- ↑ Zijlstra EE, Sande WW, Welsh O, Mahgoub ES, Goodfellow M, Fahal AH (1 January 2016). "Mycetoma: a unique neglected tropical disease". The Lancet Infectious Diseases. 16 (1): 100–112. doi:10.1016/S1473-3099(15)00359-X. ISSN 1473-3099. PMID 26738840. Archived from the original on 7 July 2020. Retrieved 30 August 2021.
- 1 2 Hamilton Bailey's Demonstrations of Physical Signs in Clinical Surgery ISBN 0-7506-0625-8
- ↑ "Dorlands Medical Dictionary:botryomycosis". 5 September 2008. Archived from the original on 5 September 2008. Retrieved 10 July 2018.
- ↑ "Skin-nontumor Infectious disorders Botryomycosis". PathologyOutlines.com, Inc. Archived from the original on 24 July 2012. Retrieved 31 July 2013.
- ↑ Capoor MR, Khanna G, Nair D, et al. (April 2007). "Eumycetoma pedis due to Exophiala jeanselmei". Indian J Med Microbiol. 25 (2): 155–7. doi:10.1016/S0255-0857(21)02178-2. PMID 17582190.
- ↑ Efared B, Tahiri L, Boubacar MS, Atsam-Ebang G, Hammas N, Hinde EF, Chbani L (2017). "Mycetoma in a non-endemic area: a diagnostic challenge". BMC Clinical Pathology. 17: 1. doi:10.1186/s12907-017-0040-5. PMC 5288886. PMID 28167862.
- ↑ "World's first clinical trial for devastating fungal disease mycetoma shows efficacy of new, promising treatment" (Press release). Drugs for Neglected Diseases initiative (DNDi). 23 November 2023.
- ↑ Johnson S (23 November 2023). "Cheap over-the-counter nail drug found to work on crippling flesh-eating disease". The Guardian.
The head of mycetoma at the DNDi labelled the discovery 'momentous', and said 'We were all very excited, it's going to be a gamechanger'.
- ↑ "Infectious Disorders (Specific Agent) Madura foot/Mycetoma/Maduramycosis". MedTech USA, Inc. Archived from the original on 22 March 2014. Retrieved 1 August 2013.