 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.016.309 |
| Chemical and physical data | |
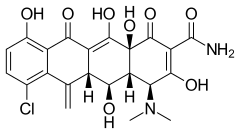
| Formula | C22H21ClN2O8 |
| Molar mass | 476.87 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Meclocycline (INN) is a tetracycline antibiotic.[1] It is used topically (i.e. for skin infections) as it is totally insoluble in water and may cause liver and kidney damage if given systemically.
Its production for medical use has been discontinued.[2] It was previously sold in the United States by Pfizer under the brand name Meclan.[3]
References
- ↑ "Meclocycline sulfosalicylate". pubchem.ncbi.nlm.nih.gov. Retrieved 3 January 2019.
- ↑ "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations". www.accessdata.fda.gov. Retrieved 3 January 2019.
- ↑ "MECLAN Drugs@FDA: FDA-Approved Drugs". www.accessdata.fda.gov. United States Food and Drug Administration. 11 August 2022. Retrieved 2022-08-11.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.