 | |
| Clinical data | |
|---|---|
| Other names | Methyl L-cysteinate |
| AHFS/Drugs.com | International Drug Names |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.017.842 |
| Chemical and physical data | |
| Formula | C4H9NO2S |
| Molar mass | 135.18 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
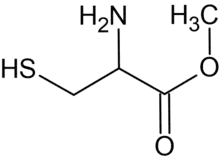
Cysteine methyl ester is the organic compound with the formula HSCH2CH(NH2)CO2CH3. A white solid, it is the methyl ester of the amino acid cysteine.
Uses
Under the brand name Mecysteine, cysteine methyl ester is a commercial drug with mucolytic activity.[1] It is used as mucolytic and fluidifying for chronic and acute respiratory disorders. The drug is sold under the commercial names Delta in Paraguay, and Pectite and Zeotin in Japan.[2]
Cysteine methyl ester is also used as a building block for synthesis of N,S-heterocycles.[3][4]
References
- ↑ Page CP (2018). "Respiratory System: Mucolytics". In Datta ST, Xiu P (eds.). Pharmacology (Fifth ed.). Philadelphia, PA: Elsevier. p. 40. ISBN 978-0-7020-7345-8.
- ↑ "Mecysteine". Drugs.com.
- ↑ Tanaka T, Nakashima T, Ueda T, Tomii K, Kouno I (June 2007). "Facile discrimination of aldose enantiomers by reversed-phase HPLC". Chemical & Pharmaceutical Bulletin. 55 (6): 899–901. doi:10.1248/cpb.55.899. hdl:10069/14910. PMID 17541189.
- ↑ Hamada Y, Shibata M, Sugiura T, Kato S, Shioiri T (1987). "New methods and reagents in organic synthesis. 67. A general synthesis of derivatives of optically pure 2-(1-aminoalkyl)thiazole-4-carboxylic acids". The Journal of Organic Chemistry. 52 (7): 1252–1255. doi:10.1021/jo00383a014.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.