 | |
 | |
| Names | |
|---|---|
| IUPAC name
Morphinan[1] | |
| Systematic IUPAC name
(4aR,10R,10aR)-1,3,4,9,10,10a-Hexahydro-2H-10,4a-(azanoethano)phenanthrene | |
| Identifiers | |
3D model (JSmol) |
|
| 1375527 | |
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
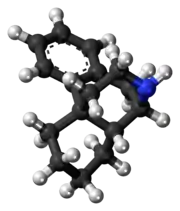
| C16H21N | |
| Molar mass | 227.351 g·mol−1 |
| Density | 1.58 g/cm3 |
| Boiling point | 115±0.05 °C (liquid oil) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Morphinan is the prototype chemical structure of a large chemical class of psychoactive drugs, consisting of opiate analgesics, cough suppressants, and dissociative hallucinogens, among others. Typical examples include compounds such as morphine, codeine, and dextromethorphan (DXM). Despite related molecular structures, the pharmacological profiles and mechanisms of action between the various types of morphinan substances can vary substantially. They tend to function either as μ-opioid receptor agonists (opioids), or NMDA receptor antagonists (dissociatives).
Structure
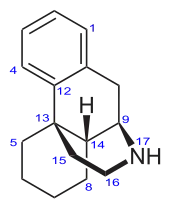
Morphinan has a phenanthrene core structure with the A ring remaining aromatic and the B and C rings being saturated, and an additional nitrogen-containing, six-membered, saturated ring, the D ring, being attached to carbons 9 and 13 of the core, and with the nitrogen being at position 17 of the composite.
Of the major naturally occurring opiates of the morphinan type—morphine, codeine and thebaine—thebaine has no therapeutic properties (it causes seizures in mammals), but it provides a low-cost feedstock for the industrial production of at least four semi-synthetic opiate agonists, including hydrocodone, hydromorphone, oxycodone and oxymorphone, and the opioid antagonist naloxone.
Structure-activity relationship
The physiological behavior of morphinans (naturally occurring and semi-synthetic derivatives) is thought to be associated with the aromatic A ring, the nitrogen-containing D ring and the "bridge" between these two rings formed by carbons 9, 10 and 11 of the core, with the D ring "above" the core (levorotatory).
Small groups are usually found on morphinan derivatives at carbons 3 and 6.
Many such derivatives have an epoxy group between carbons 4 and 5 (i.e., 4,5α-epoxy), thereby forming an E ring.
The substitution of certain bulky groups on nitrogen 17 converts an opioid agonist into an opioid antagonist, the most important of which is naloxone, a non-selective opioid antagonist with no opioid agonist properties whatsoever ("silent" antagonist). Additionally, substitution of certain very bulky groups on carbon 6 converts naloxone into a peripherally-selective opioid antagonist with no centrally-selective antagonist properties (naloxegol).
The addition of a two-carbon bridge between carbons 6 and 14 (e.g., 6,14-ethano, or 6,14-etheno), and which significantly distorts the C ring, may increase potency 1,000 to 10,000 times, or greater, compared to morphine, as in etorphine, and others. The relative potency is thought to be associated with the degree of distortion of the C ring, and is perhaps greatest in diprenorphine, where this group is α,α-dimethyl-6,14-etheno. Diprenorphine (M5050) is the recommended etorphine (M99) antagonist, but it is not a pure opioid antagonist (i.e., it is also a weak opioid agonist), so naloxone remains a significant therapeutic tool in suspected cases of opioid overdose. See also Bentley compounds.
If the D ring is "below" the core (dextrorotatory), the analgesic and euphoric properties are eliminated or are dramatically reduced, but the cough-suppressant property is retained, as in dextromethorphan.
Chemical derivatives
Immediate derivatives of morphinan include:
- 3-Hydroxymorphinan
- 3-Methoxymorphinan
- Butorphanol
- Cyclorphan
- Dextrallorphan
- Dextromethorphan
- Dextrorphan
- Dimemorfan
- Ketorfanol
- Levallorphan
- Levofurethylnormorphanol
- Levomethorphan
- Levophenacylmorphan
- Levorphanol
- Norlevorphanol
- Racemethorphan
- Racemorphan
- Oxilorphan
- Phenomorphan
- Proxorphan
- Xorphanol
More distant of derivatives include:
As well as the following:
- Morphine (and naturally occurring and semi-synthetic analogues)
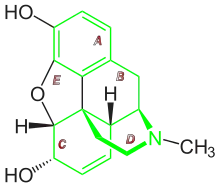

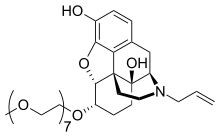
Chemical relatives
The following structures are related to morphinan:
References
- Brunton L L, Blumenthal D K, Murri N, Dandan R H, Knollmann B C. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw-Hill, 2011. ISBN 978-0-07-162442-8.
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 1522. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.