 | |
| Names | |
|---|---|
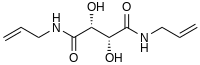
| Preferred IUPAC name
(2R,3R)-2,3-dihydroxy-N,N'-bis(prop-2-enyl)butanediamide | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.055.688 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H16N2O4 | |
| Molar mass | 228.248 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
N,N′-Diallyl-L-tartardiamide (DATD) is a crosslinking agent for polyacrylamide gels, e.g., as used for SDS-PAGE.[1] Compared to bisacrylamide gels, DATD gels have a stronger interaction with glass, and therefore are used in applications where the polyacrylamide gel acts as a "plug" structural component at the bottom of a gel electrophoresis apparatus, thereby preventing a weak discontinuous gel from sliding out from or otherwise moving within the apparatus.[1][2] Unlike bisacrylamide-polyacrylamide gels, DATD-polyacrylamide gels can be conveniently dissolved using periodic acid due to the presence of viscinal diols in DATD.[3] DATD is the slowest polyacrylamide crosslinker tested,[4] and has can act as an inhibitor of polymerization at high concentrations.[4][5][6]
See also
References
- 1 2 Baumann, Gerhard; Chrambach, Andreas (1976). "A highly crosslinked, transparent polyacrylamide gel with improved mechanical stability for use in isoelectric focusing and isotachophoresis". Analytical Biochemistry. Elsevier BV. 70 (1): 32–38. doi:10.1016/s0003-2697(76)80044-9. ISSN 0003-2697.
- ↑ Greaser, Marion L.; Warren, Chad M. (2018-11-14). "Electrophoretic Separation of Very Large Molecular Weight Proteins in SDS Agarose". Methods in Molecular Biology. New York, NY: Springer New York. pp. 203–210. doi:10.1007/978-1-4939-8793-1_18. ISBN 978-1-4939-8792-4. ISSN 1064-3745.
- ↑ Tas, Johan; de Vries, Alfons C.J.; Berndsen, RenéG. (1979). "A method for the quantitative determination of protein incorporated in solubilizable polyacrylamide gels". Analytical Biochemistry. Elsevier BV. 100 (2): 264–270. doi:10.1016/0003-2697(79)90229-x. ISSN 0003-2697.
- 1 2 Gelfi, Cecilia; Righetti, Pier Giorgio (1981). "Polymerization kinetics of polyacrylamide gels I. Effect of different cross-linkers". Electrophoresis. Wiley. 2 (4): 213–219. doi:10.1002/elps.1150020404. ISSN 0173-0835.
- ↑ Hahn, Edwin C.; Hahn, Patricia S. (1987). "Properties of acrylamide gels cross-linked with low concentrations of N,N′-diallyltartardiamide". Journal of Virological Methods. Elsevier BV. 15 (1): 41–52. doi:10.1016/0166-0934(87)90047-4. ISSN 0166-0934.
- ↑ Neumann, Ulf; Khalaf, Hosni; Rimpler, Manfred (1992). "Quantitation of proteins separated in N,N′-1,2-dihydroxyethylenebisacrylamide-crosslinkedpolyacrylamide gels". Analytical Biochemistry. Elsevier BV. 206 (1): 1–5. doi:10.1016/s0003-2697(05)80002-8. ISSN 0003-2697.