 | |
| Names | |
|---|---|
| IUPAC name
4-(methylamino)phenol sulfate | |
| Other names
N-methyl-p-aminophenol sulfate, Pictol, p-(methylamino)phenol sulfate, monomethyl-p-aminophenol hemisulfate, Metol, Elon, Rhodol, Enel, Viterol, Scalol, Genol, Satrapol, Photol. | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.216 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
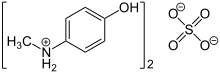
| (C7H10NO)2SO4 | |
| Molar mass | 344.38 g/mol |
| Melting point | 260 °C (500 °F; 533 K) |
| Hazards | |
| GHS labelling:[1] | |
   | |
| Warning | |
| H302, H317, H373, H410 | |
| P260, P280, P301+P312+P330 | |
| Safety data sheet (SDS) | Oxford MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Metol (or Elon) is a trade name for the organic compound with the formula [HOC6H4NH2(CH3)]2HSO4. It is the sulfate salt of N-methylaminophenol. This colourless salt is a popular photographic developer used in monochrome photography.[2]
Synthesis
Several methods exist for the preparation of N-methylaminophenol. It arises by decarboxylation of N-4-hydroxyphenylglycine (Glycin). It can be obtained by reaction of hydroquinone with methylamine.[3]
Application
Metol is an excellent developing agent for most continuous tone developer applications, and it has been widely used in published developer formulas as well as commercial products. However, it is difficult to produce highly concentrated developer solutions using Metol, and therefore most Metol developers are supplied in dry chemical mix. A developer containing both Metol and hydroquinone is called an MQ developer. This combination of agents provides greater developer activity since the rate of development by both agents together is greater than the sum of rates of development by each agent used alone (superadditivity). This combination is very versatile; by varying the quantities of Metol, hydroquinone, and restrainer, and adjusting the pH, the entire range of continuous tone developers can be made. Therefore, this form of Metol replaced most other developing agents except for hydroquinone, Phenidone (which is more recent than Metol), and derivatives of Phenidone. Notable formulas include Eastman Kodak D-76 film developer, D-72 print developer, and D-96 motion picture negative developer.
History
Alfred Bogisch, working for a chemical company owned by Julius Hauff, discovered in 1891 that methylated p-aminophenol has more vigorous developing action than p-aminophenol. Hauff introduced this compound as a developing agent. The exact composition of Bogisch and Hauff's early Metol is unknown, but it was most likely methylated at the ortho position of the benzene ring (p-amino-o-methylphenol), rather than at the amino group. Some time later, Metol came to mean the N-methylated variety, and the o-methylated variety fell out of use. Aktien-Gesellschaft für Anilinfabrikation (AGFA) sold this compound under the trade name Metol, which became by far the most common name, followed by Eastman Kodak's trade name Elon.
Because it has been in use for this purpose for over 100 years, and often by amateur photographers, there is a substantial body of evidence regarding the health problems that contact with Metol can cause. These are principally local dermatitis of the hands and forearms. There is also some evidence of sensitization dermatitis, in which repeated exposure triggers a chronic condition that is resistant to medication. The use of Metol in highly caustic solutions and the presence of other materials in darkrooms that have been implicated in dermatitis—such as hexavalent chromium salts—may exacerbate some health impacts.
References
- ↑ GHS: GESTIS 022920
- ↑ Gerd Löbbert "Photography" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a20_001
- ↑ Harger, Rolla N. (1919). "Preparation of Metol". J. Am. Chem. Soc. 41 (2): 270–276. doi:10.1021/ja01459a014.